
A new angle on Notch combination therapies
To develop cancer therapies based on modulating “ivy league” signaling pathways such as Wnt, BMP/TGFβ, Sonic Hedgehog and Notch signaling is a research avenue currently attracting considerable interest both from academia and industry. In a recent report by Ambrogio et al. in Nature Medicine (1), the authors provide evidence that a combination therapy based on simultaneously targeting the Notch signaling pathway and the DDR1 kinase may be an interesting approach towards novel therapy development for lung cancer.
The Notch signaling pathway is a cell-cell communication system controlling cellular differentiation in most, if not all, organs and tissues. The Notch pathway has a simple molecular architecture (summarized in Figure 1), yet it is able to generate quite diverse signaling outputs in a cell context-dependent manner. How the simple architecture can read cell context to produce a large array of different signaling outputs appropriate for each cell state is an area of very active research (2). In keeping with an important role in development, dysregulation of the Notch pathway is increasingly linked to disease and cancer. Mutations in genes in the core Notch pathway (Figure 1) cause a number of diseases and specific forms of cancer. In acute lymphoblastic T-cell leukemia more than 50% of the patients carry activating Notch1 mutations. In other tumor types, such as skin cancer, loss-of-function Notch mutations are causative, indicating that Notch can act as an oncogene or tumor suppressor gene, depending on the tumor type (3). In many tumor types, however, there are no direct mutations in the core pathway, but the Notch signaling output is nevertheless dysregulated and the aberrant level of Notch signaling correlates with patient prognosis. In these cases, it is conceivable that auxiliary proteins to the Notch pathway or signaling pathway interacting with Notch are dysregulated, more indirectly leading to the observed aberrant Notch signaling output. In the light of the emerging links between Notch and cancer, it comes as little surprise that finding ways to therapeutically modulate Notch signaling is a highly prioritized goal. The problem has in principle not been identifying drugs that inhibit Notch signaling: γ-secretase inhibitors for example very effectively block Notch receptor cleavage and thus downstream signaling, but as they were originally designed for systemic use, they have in most clinical trials given rise to unwanted side effects in a variety of different organs, including the gastrointestinal system, the immune system, the skin and the central nervous system, reflecting the importance of Notch in these organs. There are however several clinical trials ongoing with different dosing regimens, but there are yet no functional therapies routinely used in the clinic (3).
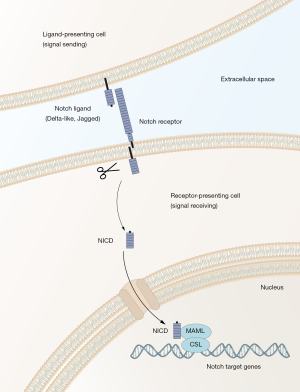
In the search for strategies to successfully modulate Notch signaling, the prospect of using combination therapies where Notch signaling and other proteins are simultaneously targeted has therefore received considerable attention, as it may alleviate some of the problems resulting from high-dose mono-therapies for Notch. In the light of this, the report by Ambrogio et al. (1) is interesting, because it provides data that the combined targeting of the kinase DRR1 and Notch signaling in lung cancer seems promising. Lung cancer is classified into small cell lung cancer (SCLC) and the more common form called non-small cell lung cancer (NSCLC), which in turn is subdivided into three histologically distinct sub-types: adenocarcinoma, squamous cell carcinoma and large cell carcinoma (4). Driver mutations are frequently found in the ErbB, ALK and KRAS genes, and patients carrying KRAS mutations have the worst prognosis and there is a dire need for therapies that reach beyond the currently used conventional chemotherapy, which is based on cisplatin/paclitaxel treatment. In the Ambrogio et al. paper, the authors searched for new strategies to intervene with adenocarcinomas, using a mouse model in which a mutated KRAS gene was conditionally activated by CRE-recombinase, leading to hyperplasias followed by more advanced adenocarcinoma. They started out by identifying genes upregulated at early stages of tumor development, i.e., the hyperplasia stage. The rationale for this was that full-blown adenocarcinomas exhibit considerable heterogeneity and the chances of identifying candidate genes that could be relevant for fueling a founder stem cell population would be higher if early tumor stages were analyzed. The DDR1 gene came out as a top candidate gene from the transcriptomic analysis. DDR1 encodes a tyrosine kinase protein, which is a member of a larger family of discoidin domain receptor 1, characterized by that they bind to and are activated by collagen and play roles in the interaction between tumor cells and the extracellular matrix (5). A functional involvement of DDR1 was demonstrated when the activated KRAS mice were crossed with DDR1−/− mice (which are phenotypically largely normal with regard to lung development), and whereas tumor formation was initiated, the medial survival time of the tumor-carrying mice was significantly extended in the absence of DDR1. Similarly, pharmacological inhibition of DDR1 reduced tumor burden, further supporting that blocking of DDR1 could be beneficial.
In addition to ErbB, ALK and KRAS mutations, nearly 10% of NSCLC patients carry activating mutations in Notch1 and 30% of the patients show loss of expression of Numb, a negative Notch regulator (6). This notion, combined with the previous report that Notch1 expression is controlled by DDR1 and that Notch1 ICD and DDR1 interact (7), focused the authors’ attention on Notch as an additional therapeutic target. Indeed, combined treatment with DDR1 and Notch inhibitors (the γ-secretase inhibitor LY-411575) was more effective in inducing apoptosis in the KRAS-driven lung adenocarcinomas than either inhibitor alone. In KRAS-activated mice in which the p53 gene was simultaneously removed, which leads to more aggressive tumor formation, mono-therapy with DDR1 and Notch inhibitors was not effective, and only the combined use of DDR1 and Notch inhibitors led to increased apoptosis in the tumors. Finally, patient-derived adenocarcinoma biopsies were grafted to the lungs of immunodeficient mice, and combined blocking of DRR1 and Notch (in this case by using an anti DLL4-antibody, demcizumab, which is in clinical trials for NSCLC) showed higher efficacy than cisplatin/paclitaxel treatment.
The elegant study by Ambrogio et al. provides an interesting new angle for Notch combination therapies and solid support for a link between DDR1 and Notch. With this said, there are however still important questions that remain unanswered. We are for example still relatively ignorant as to how DRR1 and Notch synergize. A few Notch target genes were analyzed in response to DRR1 inhibition, but whether the Notch response is quantitatively or qualitatively blunted by reduced DDR1 levels remains to be established. The use of a Dll4-blocking antibody in the xenograft experiments also leaves open the possibility that the tumor vasculature, rather than the tumor proper, was affected. The authors propose that MAPK signaling may be a common node between DRR1 and Notch, and this is an interesting concept which should be further pursued.
In addition to the DDR1-Notch combination therapy, are there other interesting combination therapies on the horizon? The fact that Notch signaling intersects with several important signaling mechanisms, such as hypoxia, Wnt and BMP/TGFβ (2) suggests that there is potential for progress on several frontiers. For example, a recent study shows that overexpression of Notch1 ICD in a variety of tumor cell lines makes inhibition of other pathways less efficient (8), arguing that hyperactivated Notch signaling negatively affects inhibitor efficacy and that lowering Notch signaling would be beneficial in combination therapies. In a mouse xenograft model, the simultaneous targeting of ErbB2 and Notch proved effective (9,10).
An important consideration for future Notch-based therapies is at which step in the signaling cascade inhibition would best be executed. As discussed above, γ-secretase inhibitors, which were also used in some of the experiments in the Ambrogio et al. paper, are effective in quenching Notch as they block cleavage of all four Notch receptors, but suffer the drawbacks of being designed for systemic use, which may lead to unwanted Notch blocking in tissues others than the tumor. An attractive idea is therefore to redesign existing γ-secretase inhibitors to decrease their distribution and make their effect more local, for example restricted only to a tumor. An alternative promising strategy to block Notch signaling is the use of antibodies that interfere with Notch ligand-receptor interaction or lock Notch receptors into a non-cleavable state. Antibody-based approaches have the advantage of being more specific, capable of targeting individual ligands or receptors rather than wiping out all Notch signaling, as is the case with γ-secretase inhibitors, and recent reports provide encouraging data from antibodies targeting the Dll4 ligand (11,12) (the Dll4 ab was used in the xenograft experiments in the Ambrogio et al. paper), the Jagged ligands (13) or Notch receptors (14). Other strategies to modulate Notch includes the use of stapled peptides mimicking Mastermind (15), a protein important for the Notch/CSL transcription complex, and small molecule inhibitors for individual Notch receptors (16). This suggests that inhibition at different levels in the signaling cascade is feasible, but recent data suggest that the CSL level maybe should be avoided, as removal/knockdown of the CSL protein leads to an unexpected tumor-promoting rather than tumor-inhibiting phenotype (17-19). The expanding array of posttranslational modifications of Notch ICDs that modulate Notch signaling output includes hydroxylation, acetylation and methylation (3) as well as phosphorylation (20,21) and these Notch-modifying proteins may serve as potentially interesting candidates for pharmacological modification and inspire new, albeit more indirect, strategies for Notch modulation.
In conclusion, the report by Ambrogio et al. is important, as it addresses a form of lung cancer which has proven difficult to combat with conventional therapies, and their discovery of a link between DDR and Notch signaling, as well as the prospect of combination therapy based on these two proteins, is interesting and inspiring.
Acknowledgments
Funding: Work in the author’s laboratory is supported by the Swedish Cancer Society, the Swedish Research Council and Knut och Alice Wallenbergs Stiftelse.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shaohua Cui (Department of Pulmonary Medicine, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.39). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ambrogio C, Gómez-López G, Falcone M, et al. Combined inhibition of DDR1 and Notch signaling is a therapeutic strategy for KRAS-driven lung adenocarcinoma. Nat Med 2016;22:270-7. [Crossref] [PubMed]
- Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development 2011;138:3593-612. [Crossref] [PubMed]
- Andersson ER, Lendahl U. Therapeutic modulation of Notch signalling--are we there yet? Nat Rev Drug Discov 2014;13:357-78. [Crossref] [PubMed]
- Pikor LA, Ramnarine VR, Lam S, et al. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer 2013;82:179-89. [Crossref] [PubMed]
- Valiathan RR, Marco M, Leitinger B, et al. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev 2012;31:295-321. [Crossref] [PubMed]
- Westhoff B, Colaluca IN, D'Ario G, et al. Alterations of the Notch pathway in lung cancer. Proc Natl Acad Sci U S A 2009;106:22293-8. [Crossref] [PubMed]
- Kim HG, Hwang SY, Aaronson SA, et al. DDR1 receptor tyrosine kinase promotes prosurvival pathway through Notch1 activation. J Biol Chem 2011;286:17672-81. [Crossref] [PubMed]
- Martz CA, Ottina KA, Singleton KR, et al. Systematic identification of signaling pathways with potential to confer anticancer drug resistance. Sci Signal 2014;7:ra121. [Crossref] [PubMed]
- Pandya K, Meeke K, Clementz AG, et al. Targeting both Notch and ErbB-2 signalling pathways is required for prevention of ErbB-2-positive breast tumour recurrence. Br J Cancer 2011;105:796-806. [Crossref] [PubMed]
- Farnie G, Willan PM, Clarke RB, et al. Combined inhibition of ErbB1/2 and Notch receptors effectively targets breast ductal carcinoma in situ (DCIS) stem/progenitor cell activity regardless of ErbB2 status. PLoS One 2013;8:e56840 [Crossref] [PubMed]
- Ridgway J, Zhang G, Wu Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature 2006;444:1083-7. [Crossref] [PubMed]
- Noguera-Troise I, Daly C, Papadopoulos NJ, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 2006;444:1032-7. [Crossref] [PubMed]
- Lafkas D, Shelton A, Chiu C, et al. Therapeutic antibodies reveal Notch control of transdifferentiation in the adult lung. Nature 2015;528:127-31. [PubMed]
- Wu Y, Cain-Hom C, Choy L, et al. Therapeutic antibody targeting of individual Notch receptors. Nature 2010;464:1052-7. [Crossref] [PubMed]
- Moellering RE, Cornejo M, Davis TN, et al. Direct inhibition of the NOTCH transcription factor complex. Nature 2009;462:182-8. [Crossref] [PubMed]
- Ye Q, Jiang J, Zhan G, et al. Small molecule activation of NOTCH signaling inhibits acute myeloid leukemia. Sci Rep 2016;6:26510. [Crossref] [PubMed]
- Kulic I, Robertson G, Chang L, et al. Loss of the Notch effector RBPJ promotes tumorigenesis. J Exp Med 2015;212:37-52. [Crossref] [PubMed]
- Procopio MG, Laszlo C, Al Labban D, et al. Combined CSL and p53 downregulation promotes cancer-associated fibroblast activation. Nat Cell Biol 2015;17:1193-204. [Crossref] [PubMed]
- Braune EB, Tsoi YL, Phoon YP, et al. Loss of CSL Unlocks a Hypoxic Response and Enhanced Tumor Growth Potential in Breast Cancer Cells. Stem Cell Reports 2016;6:643-51. [Crossref] [PubMed]
- Sjöqvist M, Antfolk D, Ferraris S, et al. PKCζ regulates Notch receptor routing and activity in a Notch signaling-dependent manner. Cell Res 2014;24:433-50. [Crossref] [PubMed]
- Santio NM, Landor SK, Vahtera L, et al. Phosphorylation of Notch1 by Pim kinases promotes oncogenic signaling in breast and prostate cancer cells. Oncotarget 2016; [Epub ahead of print]. [PubMed]


