
Should pertuzumab be used as part of neoadjuvant treatment prior to the release of the APHINITY trial results?
The 2016 publication entitled “5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial” in Lancet Oncology provided an opportunity to revisit the combination of pertuzumab and trastuzumab as a neoadjuvant treatment for HER2-positive breast cancer (1). Pertuzumab is the first Food and Drug Administration (FDA)-approved drug in the neoadjuvant setting (2). It was granted accelerated approval based on the finding that adding pertuzumab to trastuzumab plus chemotherapy increased the pathological complete response (pCR) rate from 29.0% to 45.8%. The approval raised some debate because another dual-HER2 combination—lapatinib and trastuzumab—that also showed a significantly increased pCR rate in the NeoALTTO trial did not demonstrate significantly improved survival rates in the adjuvant phase III randomized trial (ALTTO) in which the dual-HER2 lapatinib and trastuzumab combination was utilized (3). In the NeoSphere article, the combination of pertuzumab and trastuzumab with chemotherapy did not significantly improve event-free survival (EFS) [hazard ratio (HR) 0.69; 95% confidence interval (CI), 0.34–1.40] compared with trastuzumab plus chemotherapy. Interestingly, compared with NeoALTTO, NeoSphere has a numerically superior long-term EFS HR (0.69 vs. 0.78) but a lower pCR rate (45.8% vs. 51.3%) (4,5). Although no formal statistical analysis could be performed for these studies, these discordant findings may reflect the mechanistic differences between pertuzumab and lapatinib. This Commentary provides an opportunity to discuss whether we should routinely apply the dual-HER2 pertuzumab and trastuzumab combination as neoadjuvant treatment before the results of the phase III adjuvant trial (APHINITY study, NCT01358877) are available.
Does an increased pCR correlate with improved long-term survival?
In the meta-analysis of neoadjuvant studies including 11,955 breast cancer patients (CTNeoBC study), initiated by the FDA, pCR was significantly correlated with improved survival in patient-level analysis (6). However, in the trial-level meta-analysis, the correlation between pCR and long-term survival was lost: the coefficient of determination (R2) between improvements in pCR and EFS was 0.15, while that between improved pCR and overall survival (OS) was 0.08. The lack of a significant association in the trial-level analysis failed to validate that pCR is capable of predicting long-term treatment benefits on a population-wide basis; among them, more heterogeneous subgroups would be included. The authors concluded that pCR could not serve as a surrogate endpoint for improved EFS and OS in the overall breast cancer population (6). However, the lack of an association between pCR and EFS or OS may have resulted from small absolute improvement in the pCR rate (1–11%) from most neoadjuvant chemotherapy trials included in the CTNeoBC. If a dramatic increase in pCR rate could be demonstrated by neoadjuvant treatment in a certain breast cancers subgroup, there may be a higher likelihood of demonstrating a significant long-term benefit. As exemplified in in the Neoadjuvant and Adjuvant Trastuzumab In patients with HER2-positive locally advanced breast cancer (NOAH) trial, the pCR of trastuzumab plus chemotherapy was much higher than that of chemotherapy alone (38% vs. 19%, P=0.001), and the increase in pCR also reflected longer EFS in the trastuzumab arm (HR =0.64, P=0.016) (7). In a recent meta-analysis that included 36 HER2-positive neoadjuvant studies, with some studies included trastuzumab as a part of the neoadjuvant regimen, a stronger correlation between pCR rate and the corresponding EFS HR (R2 =0.63) was observed (8). In addition, in the discussion section of the NeoSphere article, Gianni et al. elegantly depicted the importance of anti-HER2 treatment in determining the association between pCR and EFS by using just eight HER2-positive breast cancer neoadjuvant clinical trials. When all eight studies were included in the analysis, the R2 between pCR and EFS was 0.25; when only four studies with at least one anti-HER2 treatment as part of the neoadjuvant treatment were chosen, the R2 between pCR and EFS increased to 0.77 (1). These meta-analyses suggested that in HER2-positive non-metastatic breast cancer, it may be worthwhile to pursue a higher pCR rate because this may more likely to be correlated with longer EFS, especially when anti-HER2 treatments are included in the neoadjuvant regimen.
Higher pCR rate by dual-HER2 combination use in the neoadjuvant setting
Under the assumption that pCR may serve as a surrogate endpoint for long-term survival, dual-HER2 inhibitor combinations by trastuzumab and lapatinib or trastuzumab and pertuzumab were brought into the neoadjuvant setting to understand their efficacy in the earlier stage of HER2-positive breast cancer. Overall, many neoadjuvant studies provided similar results that dual-HER2 inhibitor combination with chemotherapy had a significantly higher pCR rate than trastuzumab plus chemotherapy. Few clinical trials have reported long-term follow-up survival results such as EFS or OS. To our knowledge, only the survival data from three high-impact studies—NeoSphere, NeoALTTO (9), and National Surgical Adjuvant Breast and Bowel Project (NSABP) B-41 (10)—are currently available (Table 1). NeoALTTO and NSABP B-41 both utilized a combination of trastuzumab and lapatinib as the dual-HER2 inhibitors, while NeoSphere used the combination of trastuzumab and pertuzumab as dual-HER2 inhibitors. NeoALTTO and NeoSphere met the primary endpoint that pCR was significantly higher in the dual-combination and chemotherapy arm than the trastuzumab and chemotherapy arm (NeoALTTO pCR 51.3% vs. 29.5%, P=0.001; NeoSphere pCR 45.8% vs. 29.0%, P=0.014) (4,5). The difference in pCR rate was only marginal in the NSABP B-41 trial (62.0% vs. 52.5%, P=0.095), a finding that was likely related to the additional doxorubicin and cyclophosphamide used in the neoadjuvant regimen (11).

Full table
Will higher pCR with a dual-HER2 combination in the neoadjuvant setting correlate with increased long-term survival?
Although dual-HER2 blockade combinations showed an increased pCR rate in the NeoALTTO, NeoSphere, and NSABP B-41 trials, the increase did not directly translate to a significant survival benefit. In the NeoALTTO, NeoSphere, and NSABP B-41 trials, the long-term survival HR of dual blockade compared with that of trastuzumab arm was 0.78 (95% CI, 0.47–1.28), 0.69 (95% CI, 0.34–1.40), and 0.66 (95% CI, 0.34–1.25), respectively. One of the explanations for the lack of statistical significance may be attributable to the fact that these neoadjuvant clinical trials were not initially designed with adequate power to compare the survival endpoints. However, another adjuvant randomized phase III trial designed with an adequate sample size and power to evaluate the benefit of lapatinib plus trastuzumab compared with trastuzumab (ALTTO trial) failed to show that dual-HER2 blockade treatment is significantly superior to trastuzumab in terms of disease-free survival (DFS; HR, 0.84; 97.5% CI, 0.70–1.02; P=0.048) and OS (HR, 0.80; 97.5% CI, 0.62–1.03; P=0.078) (3). The claim of a statistically insignificant DFS result with a P value of 0.048 was a result of a three-arm sequential design, and to accommodate the effect of multiple comparisons, the protocol specifically stated that a P value ≤0.025 was required to claim statistical significance. Moreover, some have argued that a lower than expected number of DFS events (850 planned versus only 555 that occurred at the time of analysis) may have diminished the ability to demonstrate statistical significance (12). Nevertheless, the margin of DFS and OS benefit from the dual-HER2 combination is still less than the DFS and OS benefit of NSABP B-31 and NCCTG N9831, when trastuzumab alone was used as an adjuvant treatment compared to no HER2-based adjuvant treatment (DFS: HR, 0.60; OS: HR, 0.63) (13). Overall, more data are needed before we can substantially support that a higher pCR induced by a dual-HER2 combination in the neoadjuvant setting is directly correlated with long-term survival.
Should pertuzumab be used in the neoadjuvant setting before the adjuvant APHINITY trial results are available?
As mentioned above, the survival benefit of adding pertuzumab also did not reach statistical significance in the NeoSphere study (1). Therefore, a pertinent question is whether we should adopt the trastuzumab and pertuzumab neoadjuvant combination for any patient who needs neoadjuvant therapy. Our institute currently supports the combination use of pertuzumab and trastuzumab in the neoadjuvant setting based on the outstanding survival benefit in the metastatic setting and its immune-related mechanism that is distinct from small molecule–targeted therapy. Although both the HER2 monoclonal antibody and tyrosine kinase inhibitors (TKIs) can block the HER2-signaling pathway, only the former has shown the ability to induce long-lasting anti-tumor immunity as evidenced by the results of various trastuzumab-containing regimens with a longer or similar OS benefit compared with the PFS benefit (Table 2) (14-18). Moreover, when pertuzumab was added to trastuzumab in the metastatic setting (CLEOPATRA trial), the OS benefit margin extended further (Table 2), suggesting that the addition of pertuzumab could boost the anti-tumor immunity to a greater extent than trastuzumab alone could. Interestingly, patients with low immune marker expression but not increased HER2- or HER3-associated ligands and intracellular pathways were more likely to benefit from the pertuzumab and trastuzumab combination, suggesting a boost of anti-tumor immunity as an important mechanism of the dual-HER2 antibody combinations (19,20). Although the direct link between monoclonal antibody-induced immunity and improved survival in HER2-positive breast cancer has not been confirmed, we believe the similar phenomenon of greatly increased OS benefit by the addition of pertuzumab may herald the success of the APHINITY trial.
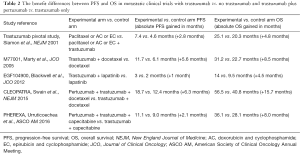
Full table
In conclusion, neoadjuvant treatment for HER2-positive breast cancer has gained popular acceptance in the past decade. Based on current evidence, trastuzumab-based chemotherapy regimen should remain the standard treatment. Dual-HER2 combination neoadjuvant treatment could increase the pCR rate, but its long-term survival benefit still requires confirmation. The pertuzumab and trastuzumab combination may have other immunogenic properties compared with HER2-TKIs that may provide durable control and extend survival; therefore, it should be a treatment option before the APHINITY trial results are available. The adjuvant APHYNITY study results will provide more crucial evidence of how to best treat our patients with HER2-positive early breast cancer.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.17). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gianni L, Pienkowski T, Im YH, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol 2016;17:791-800. [Crossref] [PubMed]
- Amiri-Kordestani L, Wedam S, Zhang L, et al. First FDA approval of neoadjuvant therapy for breast cancer: pertuzumab for the treatment of patients with HER2-positive breast cancer. Clin Cancer Res 2014;20:5359-64. [Crossref] [PubMed]
- Piccart-Gebhart M, Holmes E, Baselga J, et al. Adjuvant Lapatinib and Trastuzumab for Early Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Results From the Randomized Phase III Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization Trial. J Clin Oncol 2016;34:1034-42. [Crossref] [PubMed]
- Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 2012;379:633-40. [Crossref] [PubMed]
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25-32. [Crossref] [PubMed]
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164-72. [Crossref] [PubMed]
- Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol 2014;15:640-7. [Crossref] [PubMed]
- Broglio KR, Quintana M, Foster M, et al. Association of Pathologic Complete Response to Neoadjuvant Therapy in HER2-Positive Breast Cancer With Long-Term Outcomes: A Meta-Analysis. JAMA Oncol 2016;2:751-60. [Crossref] [PubMed]
- de Azambuja E, Holmes AP, Piccart-Gebhart M, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol 2014;15:1137-46. [Crossref] [PubMed]
- Robidoux A, Tang G, Rastogi P, et al. Evaluation of lapatinib as a component of neoadjuvant therapy for HER2+ operable breast cancer: 5-year outcomes of NSABP protocol B-41. J Clin Oncol 2016;34;abstr 501.
- Robidoux A, Tang G, Rastogi P, et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. Lancet Oncol 2013;14:1183-92. [Crossref] [PubMed]
- De Mattos-Arruda L, Shen R, Reis-Filho JS, et al. Translating neoadjuvant therapy into survival benefits: one size does not fit all. Nat Rev Clin Oncol 2016;13:566-79. [Crossref] [PubMed]
- Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 2014;32:3744-52. [Crossref] [PubMed]
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [Crossref] [PubMed]
- Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 2005;23:4265-74. [Crossref] [PubMed]
- Blackwell KL, Burstein HJ, Storniolo AM, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol 2012;30:2585-92. [Crossref] [PubMed]
- Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015;372:724-34. [Crossref] [PubMed]
- Urruticoechea A, Rizwanullah M, Im SA, et al. PHEREXA: A phase III study of trastuzumab (H) + capecitabine (X) ± pertuzumab (P) for patients (pts) who progressed during/after one line of H-based therapy in the HER2-positive metastatic breast cancer (MBC) setting. J Clin Oncol 2016;34:abstr 504.
- Baselga J, Cortés J, Im SA, et al. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J Clin Oncol 2014;32:3753-61. [Crossref] [PubMed]
- Bianchini G, Pusztai L, Pienkowski T, et al. Immune modulation of pathologic complete response after neoadjuvant HER2-directed therapies in the NeoSphere trial. Ann Oncol 2015;26:2429-36. [PubMed]


