
Tumor specific theranostic streptavidin-coupled superparamagnetic iron oxide nanoparticles for targeting therapeutic moieties in pancreatic cancer
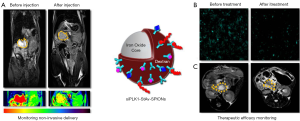
Pancreatic cancer is one of the most aggressive malignancies and burdened with a dismal prognosis (1). Given that the regulation of cell division is executed with high fidelity to maintain organ homeostasis, it is not surprising that alterations of the cell cycle are a hallmark of cancer (2). Many of the genes encoding key regulators of the cell cycle are mutated in both, sporadic and hereditary forms of cancer including pancreatic cancers implicating a role in the pathogenesis of PDAC (3). Polo-like kinases (PLKs) play an important role in the centrosome cycle which suggests that their deregulation would not be unexpected in malignant tours and their oncogenesis. PLK1 overexpression has, indeed, been observed in wide range of tumor types and was often associated with a poor prognosis (4). The use of siRNAs targeted against PLK1, which would allow a specific intervention at the molecular level could be of potential therapeutic benefit. Major hurdles of an in vivo use of such siRNAs are the adequate delivery and specific targeting, the stability of the siRNAs in serum, and the non-invasive monitoring of their effect in the tumor. Keeping in mind the limitations of classical PDAC chemotherapy and the obstacles to in vivo delivery of siRNA we have used superparamagnetic iron oxide nanoparticles (SPIONs) to develop a novel theranostic formulation that not only delivers the siRNAs in significant quantities and specifically to the tumor site but simultaneously allows for a non-invasive monitoring of the uptake by MRI. We were able to quantitate the effects on the tumor response but at the same time the relative intratumoral concentration of the therapeutic compound using MRI T2 weighted images and R2* single peak fat corrected echoes (Figure 1).
We thank Drs Klieser and Neureiter for their commentary on our paper and agree with them that systematic clinical investigations are needed to study potential adverse effects of StAv-SPIONs, a potential accumulation of SPIONs in various organs as well as a potential immunogenicity of Streptavidin (StAv). As the scope of our study was to establish a novel system for tumor specific siRNA delivery, we did not specifically study potential effects of the StAv-SPIONs carrier on the immune system. However, we measured serum cytokine concentrations, namely IL6, IL10, IL12, TNF-α, MCP1 and Interferon-γ because we anticipated a potential influence of siRNA nanoparticles on type I interferon and inflammatory cytokines in vivo. Our cytokine measurement suggested, however, no immunostimulatory properties of the siPLK1-StAv-SPIONs or of the StAv-SPIONs carrier.
Klieser and Neureiter correctly highlight further challenges to the future clinical development of this technique. In our view the first would be reducing the immunogenicity of StAv without compromising its function by means of constructing hypo-immunogenic mutants by site directed mutagenesis (6). This strategy involves replacing residues involved in B-cell epitope binding with poorly interactive residues, which would render the protein immunologically distinct from parent StAv. It has been suggested that a number of such mutations e.g. R103K, E116N, Y22S, Y83S, R84K, E101D or R103K, E116Q (6,7) would achieve this effect and render StAv less immunogenic.
Another important point we have so far not addressed in a systematic matter is, whether tumor response can be predicted from the uptake of siPLK1-StAv-SPIONs. If there would be a linear correlation between absolute concentrations of siPLK1-StAv-SPIONs initially taken up by the tumor and metastases with respect to therapeutic efficacy over time we could use the system as companion diagnostic and could stratify treatment dosage by in vivo quantification of the active substance at its site of action and evaluate it in an adaptive trial design (8). This would diminish side effects by overdosing; we could stop ineffective treatment early avoiding toxicity and thus improve quality of life.
In conclusion, we believe that by the setup of our platform we will be able to translate our findings from the mouse model to humans in due time.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Xiaotian Sun (Department of Internal Medicine, Clinic of August First Film Studio, Beijing, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.59). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Maitra A, Leach SD. Disputed paternity: the uncertain ancestry of pancreatic ductal neoplasia. Cancer Cell 2012;22:701-3. [Crossref] [PubMed]
- Zhang Y, Liu Y, Yang YX, et al. The expression of PLK-1 in cervical carcinoma: a possible target for enhancing chemosensitivity. J Exp Clin Cancer Res 2009;28:130. [Crossref] [PubMed]
- Yamaguchi T, Goto H, Yokoyama T, et al. Phosphorylation by Cdk1 induces Plk1-mediated vimentin phosphorylation during mitosis. J Cell Biol 2005;171:431-6. [Crossref] [PubMed]
- Rizki A, Mott JD, Bissell MJ. Polo-like kinase 1 is involved in invasion through extracellular matrix. Cancer Res 2007;67:11106-10. [Crossref] [PubMed]
- Mahajan UM, Teller S, Sendler M, et al. Tumour-specific delivery of siRNA-coupled superparamagnetic iron oxide nanoparticles, targeted against PLK1, stops progression of pancreatic cancer. Gut 2016. pii: gutjnl-2016-311393. [Epub ahead of print].
- Yumura K, Ui M, Doi H, et al. Mutations for decreasing the immunogenicity and maintaining the function of core streptavidin. Protein Sci 2013;22:213-21. [Crossref] [PubMed]
- Meyer DL, Schultz J, Lin Y, et al. Reduced antibody response to streptavidin through site-directed mutagenesis. Protein Sci 2001;10:491-503. [Crossref] [PubMed]
- Hatfield I, Allison A, Flight L, et al. Adaptive designs undertaken in clinical research: a review of registered clinical trials. Trials 2016;17:150. [Crossref] [PubMed]


