
The unexpected high risk of occurrence or recurrence of hepatocellular carcinoma after successful antiviral therapy with interferon-free direct-acting antivirals
Chronic hepatitis C (CHC) infection is one of the major etiologies of hepatocellular carcinoma (HCC). In the previous era of interferon-based therapy, successful viral eradication has greatly reduced the risk of disease progression and HCC occurrence (1-3). Around three quarters of HCC events may be prevented at all stages of liver fibrosis (1,4,5), even among patients with compensated liver cirrhosis (6). On the hand, the effect of interferon-based antiviral therapy in tertiary prevention of HCC recurrence is much more controversial (7-11), which may attribute to heterogeneous patient characteristics and study designs.
The innovation of direct antiviral agents (DAAs) has drastically improved the treatment efficacy with a sustained virological response (SVR) rate >90% being achieved. Viral eradication by interferon-based therapy has proved to promote fibrosis regression. Recently, It has been suggested that the application of DAA may also improve liver stiffness after short term observation (12). From the pathophysiological viewpoint of liver fibrosis, HCV eradication by means of DAA has also shown to reduce hepatic venous pressure gradient in cirrhotic patients with portal hypertension (13). Before the long-term survival benefit of DAAs could be proved, the hot issue now is its impact on HCC occurrence among CHC patients or recurrence after curative HCC treatment.
HCC occurrence among patients without previous HCC
Given that the high potency and potential fibrosis regression that DAA treatment provides, one may take HCC risk reduction by DAA for granted. Unfortunately, several recent reports did not demonstrate the expected outcome of HCC risk reduction after successful DAA therapy. A study in Italy observed that HCC occurred in 9 of 285 (3.16%) cirrhotic patients (mainly compensated) without previous HCC after DAA, despite of high SVR rate of >90% (14). Another study in England also reported a cohort of 406 decompensated cirrhotic HCV patients with an SVR rate of 78% by DAA. Although there was a reduction in incidence of hepatic decompensation among DAA-treated compared to untreated historic controls, the incidence of HCC occurrence was similar between the two groups (2.5% in months 6–15 and 4% in months 0–6 for treated patients versus 4% in untreated patients) (15).
The discrepancy of risk reduction after successful antiviral therapy between interferon-based and interferon-free DAA therapies might result from the different characteristics between the antivirals themselves or diversity of the patients enrolled. Unlike interferon, lacking immune modulation and antineoplastic effect of DAA make the issue to be elusive. Hepatic 186-gene signature has been associated with the development of HCC (16). Reversal of the signature genes after antiviral therapy might reduce the risk of HCC. Whether there exists difference in reversing the signature genes between interferon-containing and interferon free antiviral therapies needs to be explored. The most solid evidence may come from the comparison of the HCC incidence by using DAA with untreated cohort. Since it is unethical to create such a prospective study, most studies used historical untreated cohorts to answer the question. Attention should be paid due to unequal disease severities between groups. Alternatively, one may choose persistent HCV viremic patients who failed to prior interferon-based therapy as controls. It is noteworthy that it is unclear to what extend do transient application of interferon affects oncogenesis. For example, even among patients who failed interferon-based therapy, it has been suggested that the relapsers have lower risk of HCC development than non-responders (17). Similarly, the “interferon-difficult-to-treat” patients are prone to have more severe liver disease. Judging the HCC risk with DAA-treated patients should be weighed by adjusting potential confounders if the studies are to be conducted. Patients allocated to DAA may have more deteriorated liver function reserve with more advanced liver diseases that are contraindicated to interferon therapy. It may cause a higher potential of HCC occurrence in the DAA patient group. Disease severity has been always most important factors associated with HCC risk whatever among interferon-based therapy (18) or interferon-free DAA therapy (14). Taken collectively, it seems that direct comparison of HCC incidence of the SVR patients treated with DAA versus interferon will tell the truth. Until now, there is no data reported by such case-control studies. Noteworthy, it is not totally fair to compare the HCC risk between patients with interferon-based and interferon-free DAA therapy. Since all oral interferon-free DAA has only evolved for 3 years. Whether the incidence of HCC truly decreases and whether patients could benefit as much as interferon therapy do await further studies with well-controlled design and longer follow-up period.
HCC recurrence among patients with previous HCC post curative therapy (Table 1)
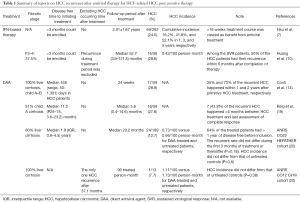
Full table
Compared with hepatitis B virus related HCC, HCV induced HCC may recur more frequently after surgical resection (21). It has been suggested that the recurrence rate was approximately 20% 1 year after surgical resection or radiofrequency ablation of HCC (22), and up to half of HCC may recur within 3 years of curative cancer treatment (21). The recurrence takes place by either intrahepatic micro-metastasis or subsequent de novo development. The discussion regarding the beneficial effect of antiviral treatment would be more complex in HCC recurrence than in HCC occurrence. Take interferon-based therapy for instance, not all studies were in agreement with the role of HCV eradication in tertiary prevention of HCC (9-11). A major issue recently is that will DAA not just have no beneficial effect, but instead do harm for HCC recurrence (14,19). The rationale may be based on the immune dysregulation during DAA therapy (23-25) that in turns promotes the reactivation of potential tumor clones.
It is imperative that mistaken definition of cured HCC due to incomplete treatment may mask the effect of antiviral treatment. HCC recurrence within 1–2 years primary cancer removal may be viewed as incomplete treatment. No matter interferon or DAA is applied, studies exploring this issue should clarify two points: how long the observation period before/after antiviral treatment is and how long the follow-up period is. Reig et al. (19) has reported a high percentage of 27.6% HCC recurrence rate 6 months after DAA. However, the median disease free time from initiating DAA treatment was 11.2 months. And 7 (43.8%) of the recurrent HCC happened <4 months between HCC treatment and last assessment of complete response. Similarly, Conti et al. (14) have noted an identical percentage of 28.8% recurrence rate sooner after DAA. The median observation period before DAA was 446 (range, 50–1,301) days in HCC patients. However, 35% and 73% of the recurrent HCC happened within 1 and 2 years after primitive HCC treatment, respectively. Both studies had short follow-up period after antiviral therapy. Given that we are talking about the risk of HCC, the comparison could only count on incidence rather than the percentage of HCC, which was lacking in both studies. Actually, another study (20) has recently shown that the incidence of HCC recurrence did not differ from that of untreated control after a median follow-up period of 20.2 months. The recurrent rate did not differ during the first 3 months of treatment or thereafter (P=0.18). The incidence rate was 0.73/100 person-months, which was not far from to the observation by Huang et al. (10) who used peginterferon/ribavirin combination therapy.
It takes more than one decade for the hepatologists to prove the benefits of interferon-based therapy toward HCV related liver and non-liver related long-term outcome. The innovation of DAA makes the elimination of HCV no longer a dream. We now have confidence of curing patients by using the very high potent weapon. The puzzle regarding liver fibrosis regression is on the way of completeness. Time is the best medicine and it would tell whether it decreases HCC occurrence, recurrence and patient survival in the near future.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Bo Zhai (Department of Hepatobiliary Surgery, the Fourth Hospital of Harbin Medical University, Harbin, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.11.10). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yu ML, Lin SM, Chuang WL, et al. A sustained virological response to interferon or interferon/ribavirin reduces hepatocellular carcinoma and improves survival in chronic hepatitis C: a nationwide, multicentre study in Taiwan. Antivir Ther 2006;11:985-94. [PubMed]
- Huang CF, Yeh ML, Tsai PC, et al. Baseline gamma-glutamyl transferase levels strongly correlate with hepatocellular carcinoma development in non-cirrhotic patients with successful hepatitis C virus eradication. J Hepatol 2014;61:67-74. [Crossref] [PubMed]
- Huang JF, Yu ML, Lee CM, et al. Sustained virological response to interferon reduces cirrhosis in chronic hepatitis C: a 1,386-patient study from Taiwan. Aliment Pharmacol Ther 2007;25:1029-37. [Crossref] [PubMed]
- Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med 2013;158:329-37. [Crossref] [PubMed]
- Yu ML, Lin SM, Lee CM, et al. A simple noninvasive index for predicting long-term outcome of chronic hepatitis C after interferon-based therapy. Hepatology 2006;44:1086-97. [Crossref] [PubMed]
- Nahon P, Bourcier V, Layese R, et al. Eradication of Hepatitis C Virus Infection in Patients With Cirrhosis Reduces Risk of Liver and Non-Liver Complications. Gastroenterology 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Hsu YC, Ho HJ, Wu MS, et al. Postoperative peg-interferon plus ribavirin is associated with reduced recurrence of hepatitis C virus-related hepatocellular carcinoma. Hepatology 2013;58:150-7. [Crossref] [PubMed]
- Singal AK, Freeman DH Jr, Anand BS. Meta-analysis: interferon improves outcomes following ablation or resection of hepatocellular carcinoma. Aliment Pharmacol Ther 2010;32:851-8. [Crossref] [PubMed]
- Tanimoto Y, Tashiro H, Aikata H, et al. Impact of pegylated interferon therapy on outcomes of patients with hepatitis C virus-related hepatocellular carcinoma after curative hepatic resection. Ann Surg Oncol 2012;19:418-25. [Crossref] [PubMed]
- Huang JF, Yeh ML, Yu ML, et al. The tertiary prevention of hepatocellular carcinoma in chronic hepatitis C patients. J Gastroenterol Hepatol 2015;30:1768-74. [Crossref] [PubMed]
- Hung CH, Lee CM, Wang JH, et al. Antiviral therapy after non-surgical tumor ablation in patients with hepatocellular carcinoma associated with hepatitis C virus. J Gastroenterol Hepatol 2005;20:1553-9. [Crossref] [PubMed]
- Knop V, Hoppe D, Welzel T, et al. Regression of fibrosis and portal hypertension in HCV-associated cirrhosis and sustained virologic response after interferon-free antiviral therapy. J Viral Hepat 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Afdhal N, Asselah T, Everson GT, et al. HCV eradication results in reduction of hepatic venous pressure gradient 48 weeks after end of treatment; final results of the study of sofosbuvir plus ribavirin in patients with cirrhosis and portal hypertension. Available online: http://www.natap.org/2016/EASL/EASL_101.htm
- Conti F, Buonfiglioli F, Scuteri A, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol 2016;65:727-33. [Crossref] [PubMed]
- Cheung MC, Walker AJ, Hudson BE, et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol 2016;65:741-7. [Crossref] [PubMed]
- Hoshida Y, Villanueva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med 2008;359:1995-2004. [Crossref] [PubMed]
- Okanoue T, Itoh Y, Kirishima T, et al. Transient biochemical response in interferon therapy decreases the development of hepatocellular carcinoma for five years and improves the long-term survival of chronic hepatitis C patients. Hepatol Res 2002;23:62-77. [Crossref] [PubMed]
- Yu ML, Huang CF, Yeh ML, et al. Time-degenerative factors and the risk of hepatocellular carcinoma after antiviral therapy among hepatic C virus patients: a model for prioritization of treatment. Clin Cancer Res 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Reig M, Mariño Z, Perelló C, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol 2016;65:719-26. [Crossref] [PubMed]
- . ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts). Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol 2016;65:734-40. [Crossref] [PubMed]
- Sasaki Y, Yamada T, Tanaka H, et al. Risk of recurrence in a long-term follow-up after surgery in 417 patients with hepatitis B- or hepatitis C-related hepatocellular carcinoma. Ann Surg 2006;244:771-80. [Crossref] [PubMed]
- Pompili M, Saviano A, de Matthaeis N, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol 2013;59:89-97. [Crossref] [PubMed]
- Serti E, Chepa-Lotrea X, Kim YJ, et al. Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function. Gastroenterology 2015;149:190-200.e2. [Crossref] [PubMed]
- Spaan M, van Oord G, Kreefft K, et al. Immunological Analysis During Interferon-Free Therapy for Chronic Hepatitis C Virus Infection Reveals Modulation of the Natural Killer Cell Compartment. J Infect Dis 2016;213:216-23. [Crossref] [PubMed]
- Meissner EG, Wu D, Osinusi A, et al. Endogenous intrahepatic IFNs and association with IFN-free HCV treatment outcome. J Clin Invest 2014;124:3352-63. [Crossref] [PubMed]


