
Interfering with Helios-induced regulatory T cell stability as a strategy for cancer immunotherapy
The molecular control of regulatory T cell (Treg) homeostasis and differentiation is not yet completely understood in particular during inflammation and tumorigenesis.
Since the identification of Tregs in 1995 (1) and Foxp3 as the Treg lineage-defining transcription factor in 2003 (2), significant advancements have been made in the knowledge of Treg biology and function. We have learned that Tregs differentiate in two different ways: either in the thymus, from CD4+ thymocytes that strongly recognize self-antigens but bypass the negative selection and develop into anergic Tregs [natural Tregs (nTregs)]; or in the periphery, from CD4+Foxp3− T cells under specific circumstances that promote conversion into Tregs [(induced Tregs (iTregs)]. Expression of the transcription factor Helios, a member of the Ikaros zinc-finger protein family, was initially reported as a distinctive marker of nTreg (3). Further studies, however, have revealed that Helios can be induced in Foxp3+ Tregs independent of their origin (4,5), and indicated the involvement of Helios expression in the definition of stability and suppression function of Tregs (6,7).
In physiological conditions, the frequency of Tregs is kept constant within a specific range. In tumor bearing individuals, instead, Treg frequency increases in the periphery and to higher extents at the tumor site, indicating the failure of the homeostatic mechanisms that normally control the amount of Tregs that need to be produced. The molecular network underlying these changes has not been fully clarified yet. Given the unfavorable prognostic impact of intra-tumor Treg accumulation, understanding how to interfere with abnormal Treg development in this condition may help identify effective strategy for cancer immunotherapy.
The work by Nakagawa et al. explores the role of Helios-mediated Treg stability in anti-tumor immunity and investigates strategies to counteract Helios expression/function for cancer immunotherapy. Building upon their previous report demonstrating the requirement of Helios expression for the maintenance of functional Tregs in models of inflammation (7), here the authors study the relevance of targeting Helios to improve anti-tumor immunity. Remarkably, the growth of both highly immunogenic (MC38 colon adenocarcinoma) and aggressive non-immunogenic (B16F10 melanoma) mouse tumor models is affected in mice with Treg-selective Helios deficiency. Importantly, in these mice, Treg frequency at the tumor site does not increase and remains comparable to that found in the periphery. Increased production of Th1-type cytokines (TNFα and IFNγ) by tumor-infiltrating Helios-deficient Tregs points to a reverse conversion of Tregs into effector T cells (Teffs) promoted by Helios loss, or vice versa to a less efficient Teff to Treg conversion in the absence of Helios. In either case, these findings validate Helios as a critical transcription factor controlling development/maintenance of functional Tregs in the tumor microenvironment. In addition, they provide the rationale to exploit strategies able to affect Helios-mediated Treg stability for cancer immunotherapy. As an approach to substantiate this hypothesis, the authors use anti-GITR agonist antibodies, which they found to be effective in dampening Treg stability, as also previously reported by our group (8). GITR stimulation with the antibody DTA-1 recapitulates the outcome achieved in mice with Treg-selective Helios deficiency, showing reduction in intra-tumor Treg frequency and stability coupled with delayed tumor growth. Even though it is still not completely clear if interfering with Helios affects a specific subset of Tregs (nTregs vs. iTregs), these results confirm the therapeutic impact of down-regulating Helios and the possibility to pharmacologically induce this effect (Figure 1).
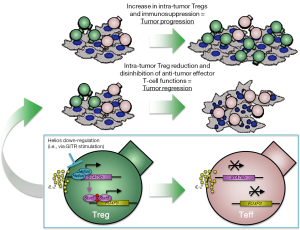
As cancer immunotherapy with agonist anti-GITR antibodies is currently under clinical investigation, understanding the molecular interplay within the GITR-Helios-Foxp3 axis will be of paramount importance for the identification of relevant pharmacodynamic and predictive biomarkers of anti-GITR therapy. In their previous report, the authors demonstrate that Helios stabilizes Tregs and Foxp3 expression by promoting STAT5 transcription (Figure 1). Investigating the relative contribution of STAT5 modulation to the anti-tumor activity of anti-GITR therapy would thus represent the logical next step toward this goal.
Acknowledgments
Funding: We would like to thank Swim Across America, Ludwig Institute for Cancer Research, Parker Institute for Cancer Immunotherapy, Center for Experimental Therapeutics at MSKCC (ETC), the Breast Cancer Research Foundation and the MSKCC Core Grant (P30 CA008748).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Chen Qian (Center for Inflammation & Epigenetics, Houston Methodist Hospital Research Institute, Houston, USA).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.11.23). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995;155:1151-64. [PubMed]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003;4:330-6. [Crossref] [PubMed]
- Thornton AM, Korty PE, Tran DQ, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol 2010;184:3433-41. [Crossref] [PubMed]
- Gottschalk RA, Corse E, Allison JP. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol 2012;188:976-80. [Crossref] [PubMed]
- Himmel ME, MacDonald KG, Garcia RV, et al. Helios+ and Helios- cells coexist within the natural FOXP3+ T regulatory cell subset in humans. J Immunol 2013;190:2001-8. [Crossref] [PubMed]
- Sebastian M, Lopez-Ocasio M, Metidji A, et al. Helios Controls a Limited Subset of Regulatory T Cell Functions. J Immunol 2016;196:144-55. [Crossref] [PubMed]
- Kim HJ, Barnitz RA, Kreslavsky T, et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science 2015;350:334-9. [Crossref] [PubMed]
- Schaer DA, Budhu S, Liu C, et al. GITR pathway activation abrogates tumor immune suppression through loss of regulatory T cell lineage stability. Cancer Immunol Res 2013;1:320-31. [Crossref] [PubMed]


