
Sleeve lobectomy or pneumonectomy for non-small cell lung cancer? Searching for an optimal balance between oncological, surgical and functional results
The main objective in surgery for non-small cell lung cancer (NSCLC) is to achieve good oncologic safety, which includes R0 resection of the tumor and radical lymphadenectomy. Patients with centrally-located NSCLC should undergo pneumonectomy (PN) but, considering the not negligible morbidity and mortality related to this technique, they could benefit from a lung tissue-preserving resection, especially when cardio-pulmonary reserve is limited. Since Thomas (1) firstly applied this procedure for therapeutic option, “sleeve” pulmonary resection was designed to conserve as much pulmonary tissue as possible.
Currently, sleeve lobectomy (SL) has an almost definite role in the surgical management of NSCLC patients whose pulmonary reserve is considered inadequate to permit PN.
On the other hand, there is an ongoing, large debate concerning the role of sleeve resection in those patients judged as “clinically-fit” for PN. Indeed, although many recent reports (2,3) have suggested that sleeve resection may achieve adequate curability rates, different opinions still emerge on the surgical completeness and early/long-term results after pulmonary sleeve resection.
In this setting, the study performed by Andersson and co-workers (4), which analysed the post-op outcomes and long-term survival results of 107 NSCLC patients who underwent SL or PN, adds very interesting information to such debate. Their results have great potential impact on the clinical decision-making process in locally-advanced NSCLC. Focusing on post-op outcomes, long-term survival and QoL results, the authors comprehensively compared these two techniques (SL vs. PN) using a propensity-matched analysis in a retrospective monocentric cohort of locally-advanced NSCLC cases. In line with other authors (5-20) (see also Table 1), they observed as PN-group was associated with higher incidence of major complications (29.9%) when compared with SL-group (P=0.027) with a remarkably different rates of re-operation (25.4% in PN-group vs. 7.5% in SL-group). Regards of the 90-day mortality rate, they observed different results between the two groups (7.5% for PN-patient vs. 5% in SL-patient) but this difference was not statistical significant. However, these results are substantially in line with data coming from a large meta-analysis focused on this topic (21). In details, the meta-analysis (including a total of 19 trials with 3,878 subjects) showed that the pooled postoperative mortality in patients undergoing SL was 2.91% (38/1,306) as compared with 5.86% (149/2,542) in patients receiving PN. Such difference resulted to be statistically significant (OR, 0.50; 95% CI, 0.34–0.72) in favour of SL-group.
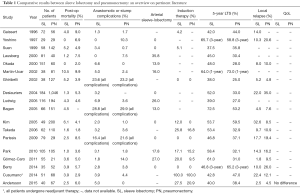
Full table
Concerning long-term survival, no difference was noted by Andersson and colleague (4). The 5-year survival rate was similar in both groups (PN: 41.8% vs. SL: 37.5%, P=0.665), this suggesting comparable oncologic results. These findings are consistent with other studies published on this topic; indeed when analysing the data coming from recent literature (see Table 1), it emerges long-term survival rates ranging from 37% to 61% after SL and from 25% and 59% after PN. In this regards, the meta-analysis performed by Shi and colleague (21) showed better long-term survival results in SL-group when compared with PN-group; in detail, the estimated combined hazard ratio (HR) for overall survival in 13 comparative studies was 0.63 (95% CI, 0.56–0.71) in favor of SL-group, and there was a statistically significant difference also.
An additional major concern when comparing SL vs. PN is the incidence of loco-regional recurrence. Andersson and co-workers (4) did not report significant differences when comparing the rates of distant metastasis or loco-regional recurrence between SL- and PN-group (P=0.798). In particular, the rates of loco-regional relapse (2.5% in SL-group vs. 7.5% in matched PN-group) were noticeably lower than those observed in literature (see Table 1) and reported in the meta-analysis (14.4% in SL patients vs. 26.1% in PN patients). The rate of loco-regional relapse is a crucial factor in evaluating the “oncological safety” of sleeve pulmonary resection as compared with PN. Such a low rate of loco-regional relapse reported by Andersson (4) is almost hard to be interpreted due to the absence of data concerning the surgical completeness, that represents an additional relevant “outcome indicator” of this surgical technique.
Indeed, in the line of extreme simplification, when comparing sleeve-resection with PN, there are several “outcome indicators” that we need to take into account. The “ideal” technique should provide for an optimal balance between oncological, surgical and functional results. In this setting, the surgical completeness stays as one of the main “outcome indicators” among with post-operative outcome, long-term survival and QoL result.
Finally, when analysing the long-term QoL results, Andersson and co-workers (4) didn’t observe any significant difference between groups in the total score. The only difference is seen for moving and breathing (better results in SL-group), but this was not statistical significant. Looking at the pertinent literature on this topic, only few studies have been reported (22,23) and results are almost preliminary. The best evidences come from a prospective analysis performed by Balduyck and colleague (22) evaluating QoL modification after SL and PN with the European Organisation for Research and Treatment of Cancer (EORTC) QoL questionnaire-C30 and LC-13. The authors prospectively enrolled 10 sleeve lobectomies and 20 pneumonectomies and questionnaires were administered before surgery and 1, 3, 6, and 12 months postoperatively. A significant higher burden of dyspnea, general pain, thoracic pain and shoulder dysfunction was observed after PN when compared with QoL data after SL. Based on these results, the authors (22) concluded that in patients with anatomically appropriate early-stage lung cancer, SL offers better quality of life than does PN. In our opinion, the recovery of a satisfactory QoL after surgery should have greater weight among the various “outcome indicators” reported above. We recently performed a detailed analysis of QoL results (questionnaires: SF-12) in a large cohort of patients after PN (23). Although we observed an overall rewarding preservation of mental and (partially) physical health, physical score (Phy-Sc) significantly decreased after PN, especially in patients with symptoms prior to surgery and with low preoperative FEV1 values. In this context, a better comprehension of the QoL evolution (before and after surgery) is needed in large prospective clinical series comparing SL and PN.
In conclusion, the advances in patient selection criteria and surgical techniques have allowed SL to evolve from a compromise to PN to “first-line” intervention for centrally located lesions of all grades. Moreover, as promising short- and long-term results were demonstrated, SL was accepted as an alternative surgical procedure to PN. Although there isn’t yet a high level of evidence, peri-operative outcomes (morbidity and mortality) favour SL. Long-term survival results did not substantially differ between SL and PN while, supposedly, QoL could be better preserved after SL as compared with PN. Recent literature has also shown evidence supporting the use of neoadjuvant treatment (24) and minimally invasive techniques (25) when performing a sleeve-resection.
Therefore, despite PN still retains a significant role in locally-advanced NSCLC, sleeve resections could be performed for centrally located tumor whenever technically, anatomically and oncologically possible.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.11.40). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thomas CP. Conservative resection of the bronchial tree. J R Coll Surg Edinb 1956;1:169-86. [PubMed]
- End A, Hollaus P, Pentsch A, et al. Bronchoplastic procedures in malignant and nonmalignant disease: multivariable analysis of 144 cases. J Thorac Cardiovasc Surg 2000;120:119-27. [Crossref] [PubMed]
- Fadel E, Yildizeli B, Chapelier AR, et al. Sleeve lobectomy for bronchogenic cancers: factors affecting survival. Ann Thorac Surg 2002;74:851-8; discussion 858-9. [Crossref] [PubMed]
- Andersson SE, Rauma VH, Sihvo EI, et al. Bronchial sleeve resection or pneumonectomy for non-small cell lung cancer: a propensity-matched analysis of long-term results, survival and quality of life. J Thorac Dis 2015;7:1742-8. [PubMed]
- Gaissert HA, Mathisen DJ, Moncure AC, et al. Survival and function after sleeve lobectomy for lung cancer. J Thorac Cardiovasc Surg 1996;111:948-53. [Crossref] [PubMed]
- Suen HC, Meyers BF, Guthrie T, et al. Favorable results after sleeve lobectomy or bronchoplasty for bronchial malignancies. Ann Thorac Surg 1999;67:1557-62. [Crossref] [PubMed]
- Yoshino I, Yokoyama H, Yano T, et al. Comparison of the surgical results of lobectomy with bronchoplasty and pneumonectomy for lung cancer. J Surg Oncol 1997;64:32-5. [Crossref] [PubMed]
- Lausberg HF, Graeter TP, Wendler O, et al. Bronchial and bronchovascular sleeve resection for treatment of central lung tumors. Ann Thorac Surg 2000;70:367-71; discussion 371-2. [Crossref] [PubMed]
- Okada M, Yamagishi H, Satake S, et al. Survival related to lymph node involvement in lung cancer after sleeve lobectomy compared with pneumonectomy. J Thorac Cardiovasc Surg 2000;119:814-9. [Crossref] [PubMed]
- Martin-Ucar AE, Chaudhuri N, Edwards JG, et al. Can pneumonectomy for non-small cell lung cancer be avoided? An audit of parenchymal sparing lung surgery. Eur J Cardiothorac Surg 2002;21:601-5. [Crossref] [PubMed]
- Ghiribelli C, Voltolini L, Luzzi L, et al. Survival after bronchoplastic lobectomy for non small cell lung cancer compared with pneumonectomy according to nodal status. J Cardiovasc Surg (Torino) 2002;43:103-8. [PubMed]
- Deslauriers J, Grégoire J, Jacques LF, et al. Sleeve lobectomy versus pneumonectomy for lung cancer: a comparative analysis of survival and sites or recurrences. Ann Thorac Surg 2004;77:1152-6; discussion 1156. [Crossref] [PubMed]
- Ludwig C, Stoelben E, Olschewski M, et al. Comparison of morbidity, 30-day mortality, and long-term survival after pneumonectomy and sleeve lobectomy for non-small cell lung carcinoma. Ann Thorac Surg 2005;79:968-73. [Crossref] [PubMed]
- Bagan P, Berna P, Pereira JC, et al. Sleeve lobectomy versus pneumonectomy: tumor characteristics and comparative analysis of feasibility and results. Ann Thorac Surg 2005;80:2046-50. [Crossref] [PubMed]
- Kim YT, Kang CH, Sung SW, et al. Local control of disease related to lymph node involvement in non-small cell lung cancer after sleeve lobectomy compared with pneumonectomy. Ann Thorac Surg 2005;79:1153-61; discussion 1153-61. [Crossref] [PubMed]
- Takeda S, Maeda H, Koma M, et al. Comparison of surgical results after pneumonectomy and sleeve lobectomy for non-small cell lung cancer: trends over time and 20-year institutional experience. Eur J Cardiothorac Surg 2006;29:276-80. [Crossref] [PubMed]
- Cusumano G, Marra A, Lococo F, et al. Is sleeve lobectomy comparable in terms of short- and long-term results with pneumonectomy after induction therapy? A multicenter analysis. Ann Thorac Surg 2014;98:975-83. [Crossref] [PubMed]
- Parissis H, Leotsinidis M, Hughes A, et al. Comparative analysis and outcomes of sleeve resection versus pneumonectomy. Asian Cardiovasc Thorac Ann 2009;17:175-82. [Crossref] [PubMed]
- Park JS, Yang HC, Kim HK, et al. Sleeve lobectomy as an alternative procedure to pneumonectomy for non-small cell lung cancer. J Thorac Oncol 2010;5:517-20. [Crossref] [PubMed]
- Gómez-Caro A, Garcia S, Reguart N, et al. Determining the appropriate sleeve lobectomy versus pneumonectomy ratio in central non-small cell lung cancer patients: an audit of an aggressive policy of pneumonectomy avoidance. Eur J Cardiothorac Surg 2011;39:352-9. [Crossref] [PubMed]
- Shi W, Zhang W, Sun H, et al. Sleeve lobectomy versus pneumonectomy for non-small cell lung cancer: a meta-analysis. World J Surg Oncol 2012;10:265. [Crossref] [PubMed]
- Balduyck B, Hendriks J, Lauwers P, et al. Quality of life after lung cancer surgery: a prospective pilot study comparing bronchial sleeve lobectomy with pneumonectomy. J Thorac Oncol 2008;3:604-8. [Crossref] [PubMed]
- Janet-Vendroux A, Loi M, Bobbio A, et al. Which is the Role of Pneumonectomy in the Era of Parenchymal-Sparing Procedures? Early/Long-Term Survival and Functional Results of a Single-Center Experience. Lung 2015;193:965-73. [Crossref] [PubMed]
- Lococo F, Cesario A, Cusumano G, et al. Sleeve lobectomy for NSCLC treatment: a simple surgical choice or a mandatory need in high-risk patients? Thorac Cardiovasc Surg 2012;60:177-8. [Crossref] [PubMed]
- Gonzalez-Rivas D, Yang Y, Sekhniaidze D, et al. Uniportal video-assisted thoracoscopic bronchoplastic and carinal sleeve procedures. J Thorac Dis 2016;8:S210-22. [PubMed]


