
Interplay between MMP-8 and TGF-β1 and its role in regulation of epithelial to mesenchymal transition in hepatocellular carcinoma
The transdifferentiation of epithelial cells into motile mesenchymal cells is a multistep biological process known as epithelial-mesenchymal transition (EMT). The epithelial to mesenchymal transition is characterized by the de-differentiation of epithelial cells to a mesenchymal-like or mesenchymal phenotype with the loss of epithelial markers such as adherence junction component E-cadherin and cytokeratin of the intermediate filament system, accompanied by the increased expression of mesenchymal proteins such as N-cadherin and vimentin. EMT is essential in embryonic development and tissue repair, and also plays a critical role in the progression of cancer where cells lose cell to cell adhesions and acquire increased motility to spread into surrounding and distant tissues (1). Accumulating evidence has demonstrated that EMT is crucial in hepatocellular carcinoma (HCC) invasion and metastasis as well as the development of anticancer drug resistance (2,3). Mechanistic studies on EMT have revealed a complex net of signaling processes under regulation (4,5). Growth factors including transforming growth factor-β (TGF-β), fibroblast growth factor (FGF), epidermal growth factor (EGF), and vascular endothelial growth factor (VEGF) participate in modulating EMT signaling (6,7). Of these regulatory factors, TGF-β is one of the most potent inducers activating EMT. The regulation of TGF-β signaling and EMT activation is a promising and active area to investigate the progression and metastasis of HCC.
In a study published recently in Cancer Letters (8), Qin and colleagues demonstrated that TGF-β1 is positively regulated by matrix metalloproteinase-8 (MMP-8), a member of the matrix metalloproteinase family that are involved in the breakdown of extracellular matrix and are identified as major modulators of tumor microenvironment contributing to HCC progression (9). A number of matrix metalloproteinases including MMP-1, MMP-2, MMP-7, MMP-9, MMP-13, and MMP-14 have been demonstrated to promote HCC invasion and metastasis (10-13). However, the role of MMP-8 in HCC progression remains unclear, and depending on malignancy types MMP-8 may exhibit opposite effects on tumor progression (14,15). In their work, Qin et al. demonstrated a positive contribution of MMP-8 in HCC progression. Moreover, they described an interesting interplay loop between MMP-8 and TGF-β1 in which these two EMT signaling modulators are reciprocally regulated contributing to the activation of EMT and HCC invasion and metastasis (Figure 1).
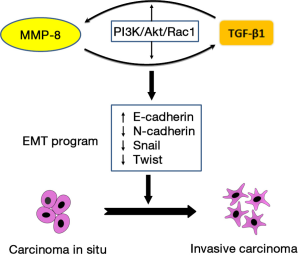
Qin et al. determined the expression of MMP-8 and TGF-β1 in several HCC cell lines including HepG2, SMMC-7721, SK-Hep-1 and HCCLM3, and normal liver cells HL-7702, and found the elevated expression of the two proteins MMP-8 and TGF-β1 was positively correlated with the invasion capacity of the studied HCC cell lines. Immunohistochemistry analysis of HCC patient specimens revealed the expression of MMP-8 and TGF-β1 was significantly higher in invasive HCC tissues than noninvasive tissues. Further, they demonstrated a positive mutual regulation between MMP-8 and TGF-β1. Depletion of MMP-8 expression with shRNA decreased TGF-β1 expression, while the overexpression of MMP-8 significantly enhanced TGF-β1 level in HCC cell lines. But how does MMP-8 regulate TGF-β1? Qin et al. analyzed PI3K/Akt/Rac1 signaling, a regulatory mechanism implicated in MMP-3 mediated malignant transformation, cell migration and invasion (16,17). They found that MMP-8 overexpression increased both Akt and Rac1 activities, whereas knockdown of MMP-8 significantly suppressed Akt and Rac1 activities, suggesting the involvement of activation of Akt and Rac1 in the mechanism of MMP-8 mediated regulation of TGF-β1. The role of PI3K/Akt/Rac1 in MMP-8-TGF-β1 regulation signaling was further substantiated by showing decreased TGF-β1 expression in cells treated with specific inhibitors of PI3K and Rac1, LY294002 and NSC23766, respectively. Intriguingly, this PI3K/Akt/Rac1 signaling is also involved in TGF-β1 mediated regulation of MMP-8. This was evidenced by the results that siRNA knockdown of TGF-β1 diminished MMP-8 expression, whereas treatment of cells with recombinant human TGF-β1 significantly enhanced MMP-8 expression. Modulation of PI3K/Akt and Rac1 activities impinged on TGF-β1-induced MMP-8 expression in a similar manner as exhibited in MMP-8 mediated TGF-β1 regulation mentioned above, further confirming a role of PI3K/Akt/Rac1 signaling in the regulation of MMP-8 expression by TGF-β1.
The finding of the bidirectional regulatory loop between MMP-8 and TGF-β1 is particularly valuable as both MMPs and TGF-β1 are key modulators regulating EMT which plays a crucial role in HCC progression. However, more mechanistic studies are needed to dissect/identify mediating molecules that promote EMT while MMP-8 and TGF-β1 are upregulated. Given this mutual regulation manner, for instance, what is the impact of combined up/down regulation of MMP-8 and TGF-β1 on Snail and Twist, two EMT-inducing transcription factors, which are independently regulated, and act cooperatively to promote EMT of HCC (18). Interestingly, the mutual regulatory mechanism does not confine to MMP-8 and TGF-β1. It has been reported that TGF-β1 also reciprocally modulates MMP-2 and MMP-9 (19). EMT regulation remains complex and new mechanisms are being found to be involved in the machinery of regulation of EMT in HCC. The mutual regulatory loop between MMP-8 and TGF-β1 can be regarded as a “new inducer” of EMT given their positive regulatory mechanism. In this regard, more functional characterizations of MMP-8 and TGF-β1 are essential to enhance the understanding of EMT in HCC. Also important is to dissect exact mechanism underlying the regulation between the two EMT modulators. Although PI3K/Akt/Rac1 signaling has been identified as a link between the mutual activation of MMP-8 and TGF-β1 (8), many other mechanisms need to be explored. For instance, it will be interesting to examine TGF-β mediated activation of Smad and non-Smad signaling pathways, which are involved in the transcriptional or non-transcriptional regulation of MMPs expression.
Importantly, it has been well demonstrated that in HCC, TGF-β1 plays a dual role in tumor development. It acts as tumor suppressor at the early stage, whereas as tumor promoter at the late stage, of the tumor development (6,20). However, the molecular mechanism underlying this functional switch of TGF-β1 remains to be elucidated. Given the mutual interplay between TGF-β1and MMP-8, it may be meaningful to further investigate the expression of these two proteins at different stages of cancer development to yield insight into the role of the mutual regulatory loop in HCC progression. In this regard, a combined approach of human and animal models may be required. Over last decades, a lot of animal models have been developed, including HCC development, metastasis and treatment. However, each model has its limitations, and exploiting proper animal models of HCC is essential.
In conclusion, the work by Qin et al. has demonstrated MMP-8 and TGF-β1 in a mutual regulatory loop as an inducer of EMT in HCC, though the detailed molecular mechanism has yet to be defined. Nevertheless, the co-expression of MMP-8 and TGF-β1 is positively associated with poor prognosis in HCC patients as demonstrated in the study by Qin et al. Further studies on the molecular mechanisms under the bidirectional regulation of MMP-8 and TGF-β1 and its role in the EMT activation may reveal important clues to the understanding of HCC progression, and the development of therapeutic intervention of HCC.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Xiao-Yu Yang (Department of special treatment II at Eastern Hepatobiliary Surgery Hospital, the Third Affiliated Hospital, Second Military Medical University (SMMU), Shanghai, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.11.68). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009;119:1420-8. [Crossref] [PubMed]
- Katsuno Y, Lamouille S, Derynck R. TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol 2013;25:76-84. [Crossref] [PubMed]
- Ma JL, Zeng S, Zhang Y, et al. Epithelial-mesenchymal transition plays a critical role in drug resistance of hepatocellular carcinoma cells to oxaliplatin. Tumour Biol 2016;37:6177-84. [Crossref] [PubMed]
- Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal 2014;7:re8. [Crossref] [PubMed]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014;15:178-96. [Crossref] [PubMed]
- Massagué J. TGFbeta in Cancer. Cell 2008;134:215-30. [Crossref] [PubMed]
- Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res 2009;19:156-72. [Crossref] [PubMed]
- Qin G, Luo M, Chen J, et al. Reciprocal activation between MMP-8 and TGF-β1 stimulates EMT and malignant progression of hepatocellular carcinoma. Cancer Lett 2016;374:85-95. [Crossref] [PubMed]
- García-Irigoyen O, Latasa MU, Carotti S, et al. Matrix metalloproteinase 10 contributes to hepatocarcinogenesis in a novel crosstalk with the stromal derived factor 1/C-X-C chemokine receptor 4 axis. Hepatology 2015;62:166-78. [Crossref] [PubMed]
- Yang MH, Chen CL, Chau GY, et al. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology 2009;50:1464-74. [Crossref] [PubMed]
- Zhao XL, Sun T, Che N, et al. Promotion of hepatocellular carcinoma metastasis through matrix metalloproteinase activation by epithelial-mesenchymal transition regulator Twist1. J Cell Mol Med 2011;15:691-700. [Crossref] [PubMed]
- Jin D, Tao J, Li D, et al. Golgi protein 73 activation of MMP-13 promotes hepatocellular carcinoma cell invasion. Oncotarget 2015;6:33523-33. [PubMed]
- Miyoshi A, Kitajima Y, Sumi K, et al. Snail and SIP1 increase cancer invasion by upregulating MMP family in hepatocellular carcinoma cells. Br J Cancer 2004;90:1265-73. [Crossref] [PubMed]
- Agarwal D, Goodison S, Nicholson B, et al. Expression of matrix metalloproteinase 8 (MMP-8) and tyrosinase-related protein-1 (TYRP-1) correlates with the absence of metastasis in an isogenic human breast cancer model. Differentiation 2003;71:114-25. [Crossref] [PubMed]
- Stadlmann S, Pollheimer J, Moser PL, et al. Cytokine-regulated expression of collagenase-2 (MMP-8) is involved in the progression of ovarian cancer. Eur J Cancer 2003;39:2499-505. [Crossref] [PubMed]
- Qian Y, Zhong X, Flynn DC, et al. ILK mediates actin filament rearrangements and cell migration and invasion through PI3K/Akt/Rac1 signaling. Oncogene 2005;24:3154-65. [Crossref] [PubMed]
- Radisky DC, Levy DD, Littlepage LE, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 2005;436:123-7. [Crossref] [PubMed]
- Wang B, Hsu SH, Majumder S, et al. TGFbeta-mediated upregulation of hepatic miR-181b promotes hepatocarcinogenesis by targeting TIMP3. Oncogene 2010;29:1787-97. [Crossref] [PubMed]
- Yang MH, Chen CL, Chau GY, et al. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology 2009;50:1464-74. [Crossref] [PubMed]
- Yang JD, Nakamura I, Roberts LR. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. Semin Cancer Biol 2011;21:35-43. [Crossref] [PubMed]


