Characterization and prognostic implications of significant blood loss during intracranial meningioma surgery
Introduction
Meningiomas consist of neoplastic arachnoidal cells imbedded in the meninges, and constitute 13–26% of primary intracranial tumors (1,2). Most meningiomas are slow growing and benign, and tend to compress or envelop the adjacent structures rather than invade them. Because a relatively clear operative plane is available, surgery aimed at total resection of the tumors is the main therapeutic option. While the surgical removal of intracranial meningiomas can reduce mass effects and may even be curative, the process is not without adverse events. In particular, meningioma resection is frequently accompanied by substantial blood loss, and preoperative embolization may be required to control surgical bleeding (3,4). This is an issue because significant surgical bleeding, i.e., ≥500 mL, is associated with morbidity and mortality in cases that undergo major non-cardiac surgery (5-7). There remains no universally accepted definition of excessive blood loss in the neurosurgical field. Moreover, the incidence of meningiomas increases with age, and elderly patients are particularly vulnerable to anemia and the detrimental effects of blood loss owing to their limited physiological reserves (8,9). Excessive bleeding during operations can also lead to the need for transfusions of red cells, platelets, or coagulation factors, thus increasing the potential for infection transmission and immunological reaction. Considering these various factors, it is important to predict significant blood loss and guide clinical management decisions in patients undergoing intracranial meningioma surgery.
In this research, we retrospectively reviewed clinical data and assessed the risk factors for significant blood loss during the resection of intracranial meningiomas. We also studied the impact of significant intraoperative bleeding on in-hospital outcomes.
Methods
Data collection
This study was conducted at a tertiary referral and 2,686-bed teaching hospital with a 20-bed neurosurgical intensive care unit (ICU) in Taiwan, Kaohsiung Chang Gung Memorial Hospital. We retrospectively collected the data of patients that had undergone craniotomy for intracranial meningioma resection from February 2009 to December 2013 after acquiring consent from the institutional review board. The cases who were treated for recurrent tumors or tissue biopsy alone were excluded from the study. In total, 99 cases were enrolled for analysis. The investigators collected the data consisting of the demographics, body mass index (BMI), preoperative laboratory examinations, Karnofsky Performance Scale (KPS), and physical status classification of American Society of Anesthesiologists (ASA). The operation details were recorded, and total intraoperative blood loss was calculated as the sum of blood in suction containers and soaked gauzes. Significant operative blood loss was defined as a loss of ≥500 mL blood volume (5-7).
Image evaluation
Magnetic resonance imaging (MRI) data of the brain were obtained preoperatively; these included T1, T2, and gadolinium enhanced-T1 sequences. The locations of the tumors were categorized as convexity, parasagittal/falx, cranial base, or posterior fossa. The site where a tumor attached to primary vascular or nervous structures, such as the cranial base or an eloquent area, was defined as a “critical location”. We calculated the size of the tumor by measuring the largest diameter of the lesion. The presence or absence of peritumoral edema was identified on T2-weighted images, and classified as absent, moderate (only peritumoral), or severe (with a shift of midline structures). In the event of a new onset of neurological deficits or for routine postoperative evaluation, follow-up images of the brain, including computed tomography scans or MRI, were performed.
Clinical management
Some of the patients received preoperative embolization for meningiomas depending on the surgeon’s decision, which was based on the size, location, and blood supply of the tumors. All cases underwent craniotomy for the removal of meningiomas, and intraoperative navigator guidance, microscopic assistance, or electrophysiological monitoring was selectively used as adjuncts to surgical resection. All specimens were obtained to establish a histological diagnosis, and the tumors were subdivided according to the World Health Organization’s classification (10). The extent of surgery was assessed using the Simpson grade of resection system (11). Postoperatively, the patients were monitored and treated in the ICU, and they received intubation with ventilation assistance over different periods depending on their neurological and medical state.
Outcome assessment
The outcomes that we focused on included the length of hospital stay, the length of postoperative ICU stay, the duration of postoperative ventilator use, and the major complications within 30 days postoperatively. The following events were defined as major complications: coma for 24 hours or longer, stroke, cardiac arrest requiring cardiopulmonary resuscitation, myocardial infarction, acute renal failure, unplanned intubation, ventilator use for 48 hours or longer, pulmonary embolism, pneumonia, bleeding requiring >4 U red cell transfusion within 72 hours after operation, deep venous thrombosis, deep or organ-space surgical site infection, sepsis, septic shock, systemic inflammatory response syndrome, and wound disruption. Death was also considered a major complication.
Statistical analysis
We analyzed data utilizing SPSS (IBM SPSS Statistics, version 20.0). Descriptive statistics were showed as mean with standard deviation (SD) or as frequencies (%). The chi-square test and Fisher’s exact test were used to compare categorical variables. The Mann-Whitney U-test and Student’s t-test were used to access continuous variables. The parameters with a P value <0.05 were entered into multivariable logistic regression model aiming to adjust for independent factors of significant bleeding during intracranial meningioma surgery. The results were showed as odds ratios (95% confidence intervals). It was considered to be significant statistically when a P value was less than 0.05.
Results
Baseline characteristics
The 99 patients who underwent craniotomy for intracranial meningiomas included 40 males and 59 females. The mean age was 60.9 (SD, 13.8; range, 20–87) years. The mean BMI was 24.5 (SD, 3.8; range, 17.6–34.6). The medical conditions included 25 patient of diabetes mellitus, 44 of hypertension, 3 of coronary artery disease, 10 of previous stroke, and 6 patients undergoing antiplatelet therapy. At admission, the mean KPS score was 68.0 (SD, 13.8; range, 20–90). The number of patients with ASA physical status classification I, II, III, and V was 3, 37, 58, and 1, respectively. The MRI scans showed 38 convexity, 22 parasagittal/falx, 27 cranial base, and 12 posterior fossa meningiomas. There were 62 tumors in a critical location. The degree of peritumoral edema was as follows: absent in 40 patients, moderate in 40 patients, and severe in 19 patients. The mean size of the tumor was 4.6 (SD 1.8; range, 1–9) cm in maximal diameter. The pathological reports showed 14 meningothelial, 7 fibrous, 14 transitional, 1 psammomatous, 2 angiomatous, 17 microcystic, 5 secretory, 1 lymphoplasmacyte-rich, 5 metaplastic, 4 chordoid, 1 clear cell, 23 atypical, 1 rhabdoid, 1 anaplastic, and 3 undetermined meningiomas. Thirty-one patients had preoperative transarterial embolization of meningiomas to facilitate surgery. The average overall length of hospital stay was 19.6 (SD 11.2; range 7–68) days.
Analysis of intracranial meningioma surgery
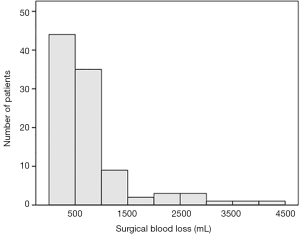
The number of cases with the Simpson resection grade 1, 2, 3, 4, and 5 was 14, 56, 12, 15, and 2, respectively. The average duration of the operation was 9.6 (SD, 3.4; range 3.9–21.1) hours. The mean intraoperative blood loss was 807.0 (SD, 806.3; range 20–4,200) mL. Figure 1 shows the distribution of the volume of surgical bleeding for the 99 patients. Significant blood loss (≥500 mL) was found in 60 (60.6%) patients. Sixty-eight patients received red cell transfusion intraoperatively. The average durations of postoperative ventilator use and length of ICU stay were 2.3 (SD 2.7; range 1–21) and 5.6 (SD 6.4; range 2–59) days, respectively.
Risk factors for significant blood loss
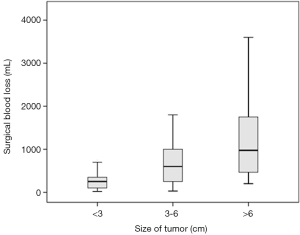
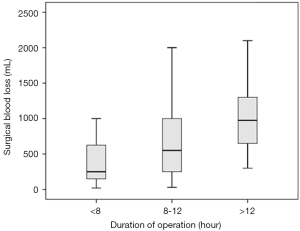
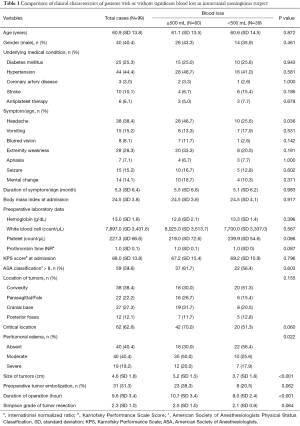
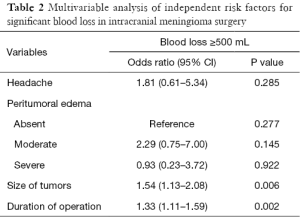
By comparing the clinical characteristics of patients with and without significant intraoperative bleeding, four significantly different parameters were identified: presentation with headache (P=0.036), the degree of peritumoral edema (P=0.022), the size of tumors (P<0.001), and the duration of the operation (P<0.001) (Table 1). These parameters were entered into multivariable regression analysis, and the factors independent for significant bleeding in intracranial meningioma surgery were tumor size [odds ratio (95% confidence interval) =1.54 (1.13–2.08); P=0.006] and duration of operation [odds ratio (95% confidence interval) =1.33 (1.11–1.59); P=0.002] (Table 2). Based on this result, we further stratified the patients into three subgroups by tumor size (i.e., <3, 3–6, and >6 cm) and operation duration (i.e., <8, 8–12, and >12 h); the relationships between these risk factors and surgical blood loss are shown in Figures 2 and 3.
Full table
Full table
Outcomes after surgery
Patients with ≥500 mL intraoperative blood loss required prolonged use of a ventilator (P=0.012), and their postoperative ICU stay and hospital stay were significantly longer than those of patients without significant blood loss (P=0.007 and P<0.001, respectively). The mean KPS score at discharge was 70.3 (SD 16.3) for patients with substantial bleeding versus 76.7 (SD 10.3) for patients without (P=0.058). The major medical complications were documented in 34 of the 99 patients within 30 days after surgery, and the overall incidence was 34.3%. The rate of 30-day complications was statistically different between the patient groups (P=0.001) (Table 3).

Full table
Discussion
The increased use of neuroimaging facilities in clinical practice and the trend for extended life expectancy have contributed to an increase in the diagnosis and treatment of intracranial meningiomas, particularly in the elderly (2,12,13). Because patient safety has become increasingly important, the complications associated with intracranial meningioma surgery should receive greater scrutiny. In the present study, the rate of significant blood loss during meningioma resection in 99 patients was up to 60.6%. These findings also indicate that significant blood loss during surgery is associated with a higher frequency of postoperative medical morbidities. Concomitantly, the duration of ventilator use and the length of ICU or hospital stay for subjects with substantial bleeding were prolonged. A trend toward lower KPS at discharge in patients with significant blood loss was also noted, although this was not statistically significant.
An increased understanding of the variables that predispose patients to postoperative morbidities is crucial for improving therapeutic outcomes. Additionally, cautious patient assessment provides an opportunity to control medical costs. A few factors related to complications or death among patients with intracranial meningioma surgery have been documented, and these include age, sex, functional status, ASA physical status classification, preoperative disseminated cancer, tumor location, peritumoral edema, tumor size, and the extent of tumor removal (12-16). However, the detrimental effects of surgical bleeding in meningioma resection have yet to be fully determined. Mortality and morbidity rates are related to bleeding during surgery, and they prominently rise for patients that lose >500 mL of blood (5). In contrast, an increase in the rate of intraoperative blood transfusion has been associated with a decrease in postoperative death for the risky population (7). In this study, a relatively large difference, 31.3%, was observed between the rates of 30-day postoperative complications in patients with or without significant blood loss. This result suggests that clinicians must pay careful attention to this determinant of surgical outcome.
Although increased surgical bleeding is associated with an increased probability of intraoperative red cell transfusion, the delivery of blood products depends not only on the amount of blood lost but also on several confounding factors, including those related to surgeons, anesthesiologists, or patients; hence, substantial variation exists among hospital practices for patients with significant surgical bleeding (7). In our opinion, risks can be identified in a direct and practical manner by calculating the volume of blood loss and evaluating the parameters related to surgical bleeding. In this study, we showed two risk factors independent for significant blood loss in intracranial meningioma surgery: the size of the tumors and duration of the operation. In other words, large tumor size and prolonged surgery in turn lead to excessive blood loss, and all these three variables probably contribute to the higher rates of morbidity.
Our analysis demonstrated that a 1-cm increase in the size of the meningioma increases the rate of significant blood loss by 53.5% (odds ratio =1.54). The blood supplied to intracranial meningiomas mainly originates from the external carotid artery, as well as the internal carotid artery, vertebral artery, or a combination of these vessels. Because meningiomas are typically slow growing, their central region is constantly supplied by feeders from the external carotid artery at the site of dural attachment (17). Unlike high-graded gliomas which exhibit rapid volume expansion and tenuous blood supply at their cores, devascularization and necrosis at the center of the meningioma is uncommon. Therefore, it is not surprising that resection of larger meningiomas results in increased blood loss.
It has been notified that duration of brain tumor surgery is an independent risk factor for extracranial complications and the potential harm of slow surgery should be of interest to neurosurgeons (18). Resection of meningiomas usually requires a standard craniotomy with extensive exposure of the lesion site to visualize the tumor and facilitate its removal. The meningioma, or even the vessel-rich dura overlying the tumor, is bloody when the raw surface of the tumor is exposed or the dura is detached from the skull. Therefore, it is a challenge for neurosurgeons to control bleeding during meningioma surgery, especially during longer lasting operations. In our study, the patients with significant blood loss underwent intracranial meningioma operations that lasted 10.7 hours on average: almost 3 h longer than in cases without significant bleeding. We reasonably doubt that the location of meningiomas is one of the predisposing factors of operative time and bleeding. Because complex or aggressive meningiomas can be deeply seated or encased by nerves and vessels, their safe resection should theoretically take more time. However, neither the location of meningiomas nor their presence in a critical location was a relevant factor for significant blood loss in this study. Another variable probably related to duration of meningioma surgery is the experience of the neurosurgeons or the quality/extent of facilities in the operating institute, but it was difficult to identify or analyze these variables in the current study.
Preoperative embolization of intracranial meningiomas is an optional procedure; in our study, 31.3% of patients underwent obliteration of at least one arterial branch of the tumor prior to surgical excision. The reported advantages of embolization of intracranial meningiomas include reducing surgical bleeding, decreasing transfusion demand, and softening the tumors to facilitate subsequent removal (17,19). In this study, there was no statistically significant difference in blood loss between patients with or without preoperative embolization. However, non-significance should not simply be interpreted as non-relation, as the variable may have a genuine influence but be undetectable because of our study design or sample size. In reality, endovascular devascularization of meningiomas is reserved for large and complex meningiomas, and only complete embolization of the tumors has an effect on intraoperative blood loss (17,20,21). It is also necessary to thoroughly assess the benefits of preoperative tumor embolization because of the additional cost involved and the increased risk of complications. In recent reports, the overall incidence of complications with preoperative meningioma embolization was 3.7–6.4% (22-24). Severe postembolization sequelae include ischemic or hemorrhagic events that can be permanent or lethal, cranial nerve palsy, scalp necrosis, and tumoral swelling leading to mass effect (17,22,23). We believe that the current evidence is insufficient to draw a firm conclusion on preoperative meningioma embolization or to fully guide its use; hence, further studies are required to investigate the benefits and risks of this adjunctive procedure.
We acknowledge that this study has several limitations that must be considered when interpreting the results. It was a retrospective review of preexisting data, and data collection through chart reviews is usually less accurate and complete than planned investigation. The number of patients recruited in the study was relatively small from a statistical standpoint; thus, it may not have had the statistical power to detect the full impact of some risk factors. Moreover, the findings reflect the experience of a solitary medical center, and parts of the surgical approaches or techniques, such as endoscopic meningioma surgery, were not executed in our hospital. Therefore, our results may not be representative of all patients that undergo intracranial meningioma surgery in other institutes. Nevertheless, despite these limitations, our data provide beneficial information for preoperative assessment and postoperative guidance of intensive care.
Conclusions
In intracranial meningioma surgery, increased tumor size and prolonged operation time increase the risk of substantial bleeding. Because significant blood loss during meningioma resection is associated with higher incidence of medical morbidities, it is important that neurosurgeons give this outcome determinant more attention.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.11.72). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Institutional Review Board of Chang Gung Memorial Hospital (104- 2001B) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bondy M, Ligon BL. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol 1996;29:197-205. [Crossref] [PubMed]
- Whittle IR, Smith C, Navoo P, et al. Meningiomas. Lancet 2004;363:1535-43. [Crossref] [PubMed]
- Rosen CL, Ammerman JM, Sekhar LN, et al. Outcome analysis of preoperative embolization in cranial base surgery. Acta Neurochir (Wien) 2002;144:1157-64. [Crossref] [PubMed]
- Raper DM, Starke RM, Henderson F Jr, et al. Preoperative embolization of intracranial meningiomas: efficacy, technical considerations, and complications. AJNR Am J Neuroradiol 2014;35:1798-804. [Crossref] [PubMed]
- Carson JL, Poses RM, Spence RK, et al. Severity of anaemia and operative mortality and morbidity. Lancet 1988;1:727-9. [Crossref] [PubMed]
- Wu WC, Smith TS, Henderson WG, et al. Operative blood loss, blood transfusion, and 30-day mortality in older patients after major noncardiac surgery. Ann Surg 2010;252:11-7. [Crossref] [PubMed]
- Wu WC, Trivedi A, Friedmann PD, et al. Association between hospital intraoperative blood transfusion practices for surgical blood loss and hospital surgical mortality rates. Ann Surg 2012;255:708-14. [Crossref] [PubMed]
- Duke M, Abelmann WH. The hemodynamic response to chronic anemia. Circulation 1969;39:503-15. [Crossref] [PubMed]
- Izaks GJ, Westendorp RG, Knook DL. The definition of anemia in older persons. JAMA 1999;281:1714-7. [Crossref] [PubMed]
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97-109. [Crossref] [PubMed]
- Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 1957;20:22-39. [Crossref] [PubMed]
- Patil CG, Veeravagu A, Lad SP, et al. Craniotomy for resection of meningioma in the elderly: a multicentre, prospective analysis from the National Surgical Quality Improvement Program. J Neurol Neurosurg Psychiatry 2010;81:502-5. [Crossref] [PubMed]
- Poon MT, Fung LH, Pu JK, et al. Outcome of elderly patients undergoing intracranial meningioma resection--a systematic review and meta-analysis. Br J Neurosurg 2014;28:303-9. [Crossref] [PubMed]
- Bateman BT, Pile-Spellman J, Gutin PH, et al. Meningioma resection in the elderly: nationwide inpatient sample, 1998-2002. Neurosurgery 2005;57:866-72; discussion 866-72. [Crossref] [PubMed]
- Sacko O, Sesay M, Roux FE, et al. Intracranial meningioma surgery in the ninth decade of life. Neurosurgery 2007;61:950-4; discussion 955. [Crossref] [PubMed]
- Bartek J Jr, Sjåvik K, Förander P, et al. Predictors of severe complications in intracranial meningioma surgery: a population-based multicenter study. World Neurosurg 2015;83:673-8. [Crossref] [PubMed]
- Dubel GJ, Ahn SH, Soares GM. Contemporary endovascular embolotherapy for meningioma. Semin Intervent Radiol 2013;30:263-77. [Crossref] [PubMed]
- Golebiowski A, Drewes C, Gulati S, et al. Is duration of surgery a risk factor for extracranial complications and surgical site infections after intracranial tumor operations? Acta Neurochir (Wien) 2015;157:235-40; discussion 240. [Crossref] [PubMed]
- Bendszus M, Klein R, Burger R, et al. Efficacy of trisacryl gelatin microspheres versus polyvinyl alcohol particles in the preoperative embolization of meningiomas. AJNR Am J Neuroradiol 2000;21:255-61. [PubMed]
- Dean BL, Flom RA, Wallace RC, et al. Efficacy of endovascular treatment of meningiomas: evaluation with matched samples. AJNR Am J Neuroradiol 1994;15:1675-80. [PubMed]
- Bendszus M, Rao G, Burger R, et al. Is there a benefit of preoperative meningioma embolization? Neurosurgery 2000;47:1306-11; discussion 1311-2. [Crossref] [PubMed]
- Borg A, Ekanayake J, Mair R, et al. Preoperative particle and glue embolization of meningiomas: indications, results, and lessons learned from 117 consecutive patients. Neurosurgery 2013;73:ons244-51; discussion ons252.
- Carli DF, Sluzewski M, Beute GN, et al. Complications of particle embolization of meningiomas: frequency, risk factors, and outcome. AJNR Am J Neuroradiol 2010;31:152-4. [Crossref] [PubMed]
- Bendszus M, Monoranu CM, Schütz A, et al. Neurologic complications after particle embolization of intracranial meningiomas. AJNR Am J Neuroradiol 2005;26:1413-9. [PubMed]