Thymic neuroendocrine tumors: an analysis of 18 cases and a literature review
Introduction
Thymic neuroendocrine tumors (TNETs), namely thymic carcinoids, are rare, and they were first described by Rosai and Higa (1) in 1972. They account for approximately 0.4% of all carcinoid tumors (2) and the reported incidence rate of TNETs is 0.02/100,000 persons per year according to the SEER database (3). Only a small number of retrospective studies are available, and the majority of related articles are case reports.
According to the 2004 World Health Organization classification of tumors (4), TNETs are included in the thymic carcinoma group, and they are classified into four entities and two major histopathologic types: well-differentiated neuroendocrine carcinomas [i.e., typical carcinoids (TC) and atypical carcinoids (AC)] and poorly-differentiated neuroendocrine carcinomas [i.e., small-cell carcinomas (SCC) and large-cell neuroendocrine]. Despite the suggestion of benign behavior implied by their name, thymic carcinoids have been noted to present a more aggressive biologic behavior than their counterparts in other sites, and they are associated with a poor prognosis (5). The overall 5- and 10-year survival rates of patients with TNETs are reported to be 28–87% and 0–61%, respectively (3,5-11). Most patients with TNETs develop local relapse or distant metastasis (DM) within 5 years of diagnosis despite receiving surgery and adjuvant treatment. To date, there are no uniform treatment strategies, because the results of different studies vary.
Here, we present 18 cases with TNETs and discuss their clinical manifestation, histopathology, treatment, and prognostic factors. A literature review of TNETs is also provided.
Methods
Patients
Between September 1993 and March 2015, 18 patients diagnosed with histologically confirmed TNETs according to the 2004 World Health Organization criteria for TNETs (4) were reviewed. This study was approved by the Institutional Review Board of Fudan University Shanghai Cancer Center, and it was conducted in accordance with the Declaration of Helsinki. The histopathology of patients with TNETs before 2004 was reviewed by experienced pathologists according to the 2004 World Health Organization criteria for TNETs (4). Clinical data were obtained from patients’ medical records and follow-up information from the referring physician or us. The sex, age, initial presentation, tumor size (TS), histological grade, Masaoka-Koga stage (MK), adjuvant treatment, resection status, and recurrence of the 18 patients were collected.
A dedicated staging system for TNETs does not exist, and different institutions worldwide use the Masaoka-Koga staging system (12), which has been shown to be a strong prognostic factor of TNETs in some studies (10,13). Thus, Masaoka-Koga staging was used in this study.
According to the International Thymic Malignancy Interest Group’s recommendation (14), surgery was defined as radical when complete tumor resection (R0) was performed with negative gross and microscopic margins. Incomplete resection was defined when there was a microscopically residual disease (R1) or macroscopically residual disease (R2). In our cases, we defined biopsy as no surgery. The grade of TNETs was divided into well-differentiated and poorly differentiated tumors. Well-differentiated tumors include TC and AC. Poorly differentiated tumors include large-cell neuroendocrine carcinoma and small-cell carcinoma (4).
The TS was determined from the surgical specimen or by chest computed tomography examination when the tumor was not resected.
Statistical analysis
Statistical analysis was performed by using SPSS, version 17 software (SPSS Inc., Chicago, IL, USA). Overall survival (OS) was defined as the time interval between the date of surgery or the last day of initial treatment [chemotherapy (CT) or radiotherapy (RT)] and the date of death or the date of the last follow-up. Progression-free survival (PFS) was defined as the time interval from the date of surgery or the last day of initial treatment (CT or RT) to the date of recurrence, including local relapse and distance metastasis, or death, whichever comes first.
Survival curves were calculated by the Kaplan-Meier method commencing from the date of surgery or the last day of initial treatment (CT or RT). The log-rank test was used to compare OS and PFS between the different factors, including sex, age, tumor stage, TS, tumor grade, adjuvant therapy, and surgery status. Confidence intervals (CI) were calculated at the 95% level with two-sided statistical tests, and P<0.05 was considered statistically significant.
Results
Clinical characteristics
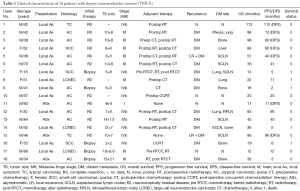
The clinical characteristics, treatment, and outcome of individual patients are summarized in Table 1. The age of our patients at the time of diagnosis ranged from 18–70 years, with a median age of 48 years. Thirteen of 18 patients (72.2%) were male. Four of 18 patients (22.2%) were asymptomatic and had their tumor discovered on routine chest radiography examination. Fourteen of 18 patients (77.8%) presented with local symptoms related to the mediastinal mass, ranging from chest discomfort and cough to cervical lymph node enlargement and superior vena cava syndrome. None of the patients had clinical manifestations of abnormal hormone secretion, such as full moon face, sanguine temperament appearance, centripetal obesity, acne, purple lines, high blood pressure, secondary diabetes and osteoporosis, etc. Based on the Masaoka-Koga staging system (12), 8 of 18 patients (44.4%) were stage III (invasion into neighboring organs); 9 of 18 (50%) were stage IV [metastasis to the supraclavicular lymph nodes (SCLN), liver, lung, and bone]; and only 1 (5.6%) was stage I (macroscopically completely encapsulated tumor).
Full table
The histology of TC and AC was regarded as well differentiated (n=12/18 patients; 66.7%), and the histology of large-cell neuroendocrine carcinoma and small-cell carcinoma was regarded as poorly differentiated (n=6/18 patients; 33.3%).
As for the neuroendocrine markers, the positive rates of synaptophysin, chromogranin A, neuron-specific enolase and CD56 were 94.4%, 88.9%, 100% and 100%, respectively. The range of Ki67 was 1–50%.
Treatment
The majority of patients (72.2%) underwent surgical resection: 7(53.5%) underwent R0 resection and 6 (46.2%) underwent R2 resection. Five of 18 patients (27.8%) had undergone a biopsy because of unresectable tumors or distant metastasis (DM). Five patients (27.8%) who underwent biopsy were firstly treated with CT, RT, or supportive care.
RT was administered in 14 patients (77.8%), most of whom received RT as an adjuvant treatment postoperatively (10/14 patients; 71.4%). Four patients received RT as radical treatment. The median total dose of RT was 60 Gy (range, 50–64 Gy). The RT technology was 3-dimensional conformal RT or intensity-modulated RT. Only one patient received RT with 2-dimensional irradiation technology.
Thirteen patients (72.2%) received CT, mostly adjuvant treatment postoperatively (7/13 patients; 53.8%). One patient received CT preoperatively, four received chemoradiotherapy, and one received CT only. All the patients with adjuvant CT used agents being cisplatin + etoposide. The five patients without surgery received cisplatin + etoposide (3/5 patients, 60%), cisplatin + etoposide +ifosfamide (1/5 patients, 20%) or temozolomide + capecitabine (1/5 patients, 60%) as first line treatment. Only one patient received cisplatin + etoposide as neoadjuvant CT before surgery.
Survival analysis and prognostic factors
The median follow-up duration was 40 months (range, 11–112 months). Sixteen of 18 patients (88.9%) had experienced complete follow-up, and two of 18 patients lost follow-up at the time of 20 and 11 months, respectively. Five patients died at the last follow-up due to DM. Fourteen patients (77.8%) had a DM [lung, 4 patients; supraclavicular lymph nodes (SCLN) or retroperitoneal lymph node (RPLN), 7 patients; pleura, 1 patient; bone, 4 patients; liver, 1 patient] after initial treatment, and 2 of 7 patients (28.6%) had local relapse after R0 resection. All of the lung metastasis was discovered by chest CT without any symptoms. The supraclavicular lymph node metastasis was discovered by neck CT with short axis exceeding 1 cm or physical examination. The RPLN, liver and pleura were discovered by abdominal CT without any symptoms. The four patients with bone metastasis have a pain before the bone metastasis was discovered and they were diagnosed through X-ray. Three patients had DM before initial treatment (patient 7 and 9 had liver metastasis; patient 16 had lumbar vertebrae bone metastasis).
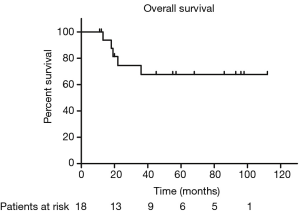
The median PFS duration and disease-free survival (DFS) duration were 41 months (95% CI, 4.75–77.25 months) and 60 months (95% CI, 37.37–82.63 months), respectively. Because more than half of patients were still alive, we couldn’t calculate the median OS and 95% CI. The 5-year OS and 5-year PFS rates were 67.7% and 37.9%, respectively (Figures 1,2). The 5-year DFS rate in the patients with R0 resection was 66.7% (Figure 3).
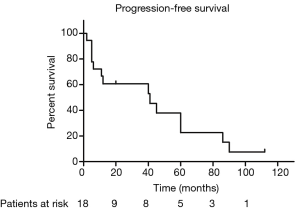
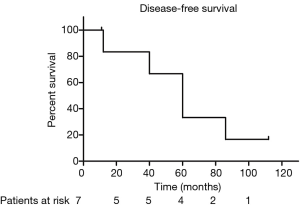
In univariate analysis, the surgical status (R0, R2 vs. no surgery, P=0.018), tumor grade (well-differentiated vs. poorly differentiated, P=0.028) were statistically significant predictors of OS and the surgical status (R0, R2 vs. no surgery, P=0.018) was also statistically significant predictors of PFS. Sex, age, tumor stage, TS and adjuvant therapy were not statistically significant (Tables 2,3). We could not perform multivariate analysis because of the small sample size.
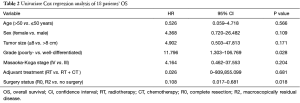
Full table
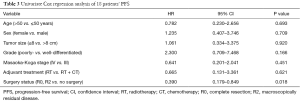
Full table
Discussion
TNETs are rare, with about 400 cases reported in the literature to data (10). The fact that 13 patients (72.2%) were men indicated male predominance, which was consistent with the findings of other reports (3,10,13). In addition, we found that patients with TNET had nonspecific symptoms when the tumor was discovered. Thus, when a mediastinal mass is present, TNETs should be considered. Neuroendocrine markers play an important role in the differential diagnosis (15). Thymomas are negative for neuroendocrine markers such as synaptophysin or chromogranin A, and mediastinal paragangliomas are usually negative for CAM 5.2 cytokeratins.
No official TNET stage classification has been defined by the Union for International Cancer Control. However, the Masaoka-Koga staging system has been widely used in previous studies. Some studies (10,13) have reported that the tumor stage plays an important role in prognosis. We found that a majority of patients with TNET (17 of 18 patients; 94.4%) presented at an advanced stage (III or IVb), which indicated the highly aggressive biological behavior of this type of tumor. Although some studies (10,13) have shown an obvious difference in OS between the early stage (I and II) and advanced stage (III and IV), there was no statistical significance between stage III and stage IVb in our analysis.
Whether the grade of tumor affects the survival time is uncertain. In general, high-grade tumors have higher biologic aggressiveness, which result in a poor prognosis. However, Filosso et al. (10) reported that the grade did not influence patients’ survival time. In contrast, another study (13) showed that the histological grade was an independent prognostic factor of OS. We also found a significant difference between well-differentiated tumors and poorly differentiated tumors in terms of OS (P=0.006).
We reviewed patients’ treatment and survival time in published series. The 5-year survival rates varied and ranged from 28–84% (Table 4). In our study, the 5-year OS rate was 67.7%, which suggests that the patients may have received a better treatment strategy. In addition to surgery, formal adjuvant therapy may benefit patients.
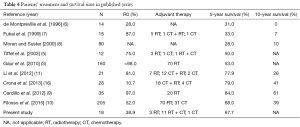
Full table
Filosso et al. (10) conducted the largest study on 205 patients diagnosed with TNETs, and they reported that the ability to undergo surgery and the completeness of resection were the strongest prognostic factors in both univariate and multivariate models. Our results also showed that patients who underwent R0 or R2 resection had a better OS and PFS than those who only underwent biopsy (P<0.05). Yet, there was no difference between patients who underwent R0 and R2 resection. Therefore, the main prognostic factor is surgical resection. The only patient who survived and was free of disease at 112 months (nearly 10 years) underwent total excision, regardless of the tumor grade. Thus, TNETs should be subjected to radical surgical resection whenever feasible, as the European Society for Medical Oncology guidelines suggest, and sometimes surgical excision of local relapse is required (17).
Concerning adjuvant treatment, Tiffet et al. (5) reported a better outcome (no local relapse) for patients who received RT after complete tumor resection. However, Filosso et al. (10) did not find any statistical advantage in OS for adjuvant CT or RT in both univariate and multivariate models. Conversely, Gaur et al. (3) found that patients who underwent RT did worse than those who did not undergo RT. Although the role of CT and RT in patients with TNET has not yet been established, a neoadjuvant treatment has been advocated with the aim to achieve tumor shrinkage, making R0 resection possible in some clinical experiences (6,9).
Although the patients with TNET experienced recurrence [local recurrence (LR) or DM] postoperatively, they survived for a long time after recurrence. The median survival time from recurrence to the date of death or the date of the last follow-up was 14 months (95% CI, 8.7–19.3 months). Treatment strategies included RT, CT, and reoperation. Therefore, active treatment after recurrence is still greatly beneficial to patients.
In recent years, an increasing number of clinical trials about targeted therapy of neuroendocrine tumors (NETs) have been conducted. Targeted therapy such as everolimus or sunitinib has led to a significant increase in PFS in patients with pancreatic NETs (18,19). Likewise, everolimus is effective for advanced lung or progressive gastrointestinal tract neuroendocrine tumors, and it is associated with an almost 3-fold improvement in PFS (11 vs. 3.9 months), which corresponds with a reduction in the risk of disease progression or death by 52% compared with a placebo (20). However, there has not yet been a prospective study on the targeted therapy of TNET since it is rare. Grande et al. (21) reported clinical activity in patients with advanced NETs, regardless of previous treatment with a median PFS of 9.5 months (3.4 months for lung and thymic NETs). Therefore, targeted therapy is a promising treatment in the future, and new evidence should be found.
In conclusion, this study was limited by its retrospective design and small sample size. Adequate surgical resection is a strong prognostic factor for NETs. Adjuvant RT may contribute to controlling LR to improve OS and PFS and adjuvant CT could not bring benefit for patients. According to the MK, there was no statistical significance between the advanced tumor stages (III vs. IVb) in regard to OS and PFS. Patients with well-differentiated TNET have a better OS. In addition, active treatment after recurrence will greatly benefit patients’ OS.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.11.77). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Fudan University Shanghai Cancer Center (ZRB1610165-6) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rosai J, Higa E. Mediastinal endocrine neoplasm, of probable thymic origin, related to carcinoid tumor. Clinicopathologic study of 8 cases. Cancer 1972;29:1061-74. [Crossref] [PubMed]
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [Crossref] [PubMed]
- Gaur P, Leary C, Yao JC. Thymic neuroendocrine tumors: a SEER database analysis of 160 patients. Ann Surg 2010;251:1117-21. [Crossref] [PubMed]
- Travis WD, Brambilla E, Müller-Hermelink HK, et al. editors. Pathology & Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer (IARC) Press, 2004:145-247.
- Tiffet O, Nicholson AG, Ladas G, et al. A clinicopathologic study of 12 neuroendocrine tumors arising in the thymus. Chest 2003;124:141-6. [Crossref] [PubMed]
- de Montpréville VT, Macchiarini P, Dulmet E. Thymic neuroendocrine carcinoma (carcinoid): a clinicopathologic study of fourteen cases. J Thorac Cardiovasc Surg 1996;111:134-41. [Crossref] [PubMed]
- Fukai I, Masaoka A, Fujii Y, et al. Thymic neuroendocrine tumor (thymic carcinoid): a clinicopathologic study in 15 patients. Ann Thorac Surg 1999;67:208-11. [Crossref] [PubMed]
- Moran CA, Suster S. Neuroendocrine carcinomas (carcinoid tumor) of the thymus. A clinicopathologic analysis of 80 cases. Am J Clin Pathol 2000;114:100-10. [Crossref] [PubMed]
- Cardillo G, Rea F, Lucchi M, et al. Primary neuroendocrine tumors of the thymus: a multicenter experience of 35 patients. Ann Thorac Surg 2012;94:241-5; discussion 245-6. [Crossref] [PubMed]
- Filosso PL, Yao X, Ahmad U, et al. Outcome of primary neuroendocrine tumors of the thymus: a joint analysis of the International Thymic Malignancy Interest Group and the European Society of Thoracic Surgeons databases. J Thorac Cardiovasc Surg 2015;149:103-9.e2. [Crossref] [PubMed]
- Li GA, Liu T, Cai BQ. Clinical features of thymic neuroendocrine carcinoma: analysis of 21 cases. Zhonghua Zhong Liu Za Zhi 2012;34:382-4. [PubMed]
- Koga K, Matsuno Y, Noguchi M, et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int 1994;44:359-67. [Crossref] [PubMed]
- Song Z, Zhang Y. Primary neuroendocrine tumors of the thymus: Clinical review of 22 cases. Oncol Lett 2014;8:2125-2129. [PubMed]
- Huang J, Detterbeck FC, Wang Z, et al. Standard outcome measures for thymic malignancies. J Thorac Oncol 2010;5:2017-23. [Crossref] [PubMed]
- Lausi PO, Refai M, Filosso PL, et al. Thymic neuroendocrine tumors. Thorac Surg Clin 2014;24:327-32. [Crossref] [PubMed]
- Crona J, Björklund P, Welin S, et al. Treatment, prognostic markers and survival in thymic neuroendocrine tumours. a study from a single tertiary referral centre. Lung Cancer 2013;79:289-93. [Crossref] [PubMed]
- Öberg K, Hellman P, Ferolla P, et al. Neuroendocrine bronchial and thymic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:vii120-3. [PubMed]
- Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514-23. [Crossref] [PubMed]
- Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501-13. [Crossref] [PubMed]
- Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 2016;387:968-77. [Crossref] [PubMed]
- Grande E, Capdevila J, Castellano D, et al. Pazopanib in pretreated advanced neuroendocrine tumors: a phase II, open-label trial of the Spanish Task Force Group for Neuroendocrine Tumors (GETNE). Ann Oncol 2015;26:1987-93. [Crossref] [PubMed]