
Low frequency ultrasound combined with baicalein can reduced the invasive capacity of breast cancer cells by down regulating the expression of MMP-2, MMP-9, and u-PA
Breast cancer is the most common carcinoma and is considered as a major cause of cancer related death of women worldwide (1-3). Multiple factors that include a switch to western diet and increased stress (4) are believed to be associated with the initiation and progression of breast cancer (5,6). In China, the breast cancer incidence is relatively lower than that in the western countries, such as the United States (7). However, predictive models strongly suggest that breast cancer will soon reach epidemic proportions (8). Over the past three decades, many patients have died owing to breast cancer metastasis and recurrence. Therefore, there is an urgent need to develop effective and comprehensive strategies for the treatment of breast cancer.
Earlier studies have demonstrated that radiation with low frequency ultrasound can reduce DNA and protein synthesis, and reduce the invasive capacity of several types of cancer cells, including breast cancer cells (9). It has also been reported that baicalein, the active component of Chinese herbal medicine (skullcaps) can inhibit the migration and metastasis of breast cancer by arresting cell proliferation and promoting cell apoptosis (10). However, the exact mechanism underlying these effects has not been well understood. Furthermore, the effect of combined treatment with low frequency ultrasound and baicalein on the invasive capacity of breast cancer cells has not been reported.
In the current study, we examined the inhibitory effect of the combination of low frequency ultrasound and baicalein on the invasive activity of breast cancer cell line MDA-MB-231 and explored the potential underlying mechanism. The findings in this report will greatly aid the identification of potential targets for the development of novel and effective strategies for breast cancer treatment and prevent the metastasis and recurrence of breast cancer.
Methods
Cell line
The human breast cancer cell line MDA-MB-231 (ER–, PR–, HER2–) used in this study was purchased from ATCC. MDA-MB-231 cells are highly invasive and belong to the basal-B subtype. MDA-MB-231 cells were cultured in RPMI 1640 medium (Gibico, USA), supplemented with 10% FBS (Gibico, USA) and 1% penicillin/streptomycin (Gibico, USA) in a humidified incubator at 37 °C in 5% CO2, and harvested with 0.25% trypsin–0.02% EDTA (Sigma, USA).
The cells were divided into four groups: low frequency ultrasound group, baicalein group, combination group and control group. Cells in the low frequency ultrasound group were irradiated with ultrasound using 838A ultrasonic therapy (SXUltrasonic biotech. CN). The probe was fixed on the bracket with the top side as the irradiation surface. Cells were placed in a centrifuge tube, and irradiated for 5 min at room temperature at a frequency of 840 kHz, 0.75 W/cm2. Cells in the baicalein group were treated with baicalein at a final concentration of 150 µmol/L for 24 h. Cells in the combination group were first irradiated for 5 min at room temperature at a frequency of 840 kHz and an intensity of 0.75 W/cm2, followed by baicalein treatment at a final concentration of 150 µmol/L for 24 h. Cells in the control group received no treatment.
Transwell migration assay
Cells (1×105/well) in serum free RPM1640 were plated into the top chamber coated with 150 mg of Matrigel (BD Biosciences, USA). The bottom chamber contained 500 µL medium supplemented with 10% FBS. After incubation at 37 °C in 5% CO2 for 24 h, the inserts were washed with water and the cells remaining on the upper surface of the membrane were removed with a cotton swab. The cells that migrated through the 8-mm sized pores and adhered to the lower surface of the membrane were fixed with 4% paraformaldehyde, stained with 0.1% crystal violet and photographed. Five fields were counted under a microscope (Olympus, JPN). All assays were independently repeated thrice. The efficacy of the inhibition of invasion was calculated using the following formula:
Inhibitory efficacy (%)=(1 − invasive cell counts in observation group/invasive cell counts in control group) × 100%.
Total RNA extraction
Total RNA from cultured cells was extracted with Trizol (Applied Science, Germany) according to the manufacturer’s instructions. Briefly, cells were homogenized in 1 mL Trizol, transferred to a 1.5-mL RNase free tube, and incubated at RT for 5–10 min at which time, 0.2 mL Chloroform (Sigma, USA) was added to the tube and vortexed for 15 s. The mixture was allowed to sit at RT for 2–3 min before centrifuging at 12,000 g for 10 min at 4 °C. The aqueous layer (400 µL) was transferred to a new RNase free tube, mixed with 0.4 mL isopropyl alcohol, allowed to stand at room temperature for 10 min to precipitate the RNA, and then centrifuged at 12,000 g for 5 min at 4 °C. The RNA pellet was washed with 75% EtOH. Finally, the pellet was air dried and re-suspended in DEPC H2O. RNA purity and concentration were assayed using NanoDrop ND-1000 (NanoDrop Technologies, USA).
Real-time PCR
cDNA was synthesized using 2 µg of RNA and PrimeScript RT Reagent Kit (Takara, JPN) following the manufacturer’s instructions. Real-time PCR assay was performed in a ABI 7500 thermal cycler (ABI, USA) using SYBR Premix Ex Taq (Takara, JPN). The final real-time PCR mixture of 20 µL in a capillary tube comprised 2 µL cDNA, 10 µL SYBR Green Master Mix, 0.5 µL each of forward and reverse primers (10 pmol), and 7 µL of nuclease-free water. Melting curves were analyzed to confirm the synthesis of a single PCR product of each primer. GAPDH was amplified as an endogenous control and the fold change in expression of each target mRNA relative to that of GAPDH was calculated based on 2−∆∆Ct relative expression formula: . The primer sequences of forward and reverse primers are shown in Table 1.

Full table
Protein extraction
Cells were homogenized in 400 µL RIPA for extracting total protein. The homogenate was subjected to sonication on ice for about 5 min, allowed to stand on ice for 30 min and then centrifuged for 10 min at 4 °C. The process was repeated one more time and protein concentration was determined by BCA. The extract containing 5 µg/µL protein was stored at −80 °C till further use.
Western-blot
Twenty-five micrograms of extracted proteins from each group were subjected to 10% denaturing SDS-PAGE and blotted onto PVDF membrane (400 mA, 90 min). The membranes were blocked with 1% BSA in TBST buffer for 30 min at 30 °C. Specific antibodies for matrix metalloproteinase 2 (MMP-2) (Santa Cruz, USA), MMP-9 (Abcam, USA), urokinase type plasminogen activator (u-PA) (Santa Cruz, USA) and GAPDH (Abcam, USA) were used as the primary antibodies at 1:2,000 dilution and incubated at 4 °C overnight. After washing thrice with TBST buffer (10 min/wash), the membranes were reacted with HRP-conjugated IgG as secondary antibody (Santa, USA) at 1:5,000 dilution and incubated at 37 °C for 1 h. After the final wash, the membranes were developed using Western Lightning ECL. Gray values of the bands were measured with Image J (NIH, USA) to obtain semi quantitative protein expressional levels.
Statistical analysis
Statistical analysis was conducted using SPSS 19.0 statistical package. All data were presented as mean ± SD. One-way ANOVA with Tukey HSD post hoc t-tests were used to analyze the differences in gene expressions between different groups. A P value of less than 0.05 was considered significant.
Results
Reduced invasive activity of MDA-MB-231 cells
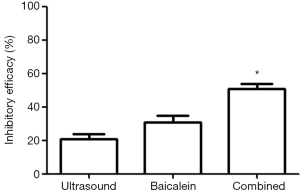
In our study, the invasive activity of MDA-MB-231 cells was reduced significantly after different treatments (Figure 1). Specifically, the invasive activity was reduced by 21.6%±1.68% after low frequency ultrasound radiation, 31.9%±2.19% after baicalein treatment, and 51.6%±2.01% after combined treatment with low frequency ultrasound and baicalein. As can be seen from these results, the reduction in the invasive activity in the combination group was highest when compared with that in either ultrasound group or baicalein group (P<0.01) (Figure 2).
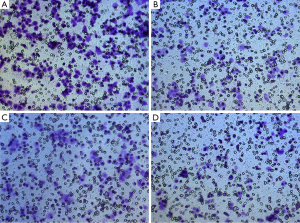
Reduced expression of MMP-2, MMP-9, and u-PA mRNA
RT-PCR was conducted to examine and analyze the changes in the expression of MMP-2, MMP-9, and u-PA at transcriptional level before and after treatment. As shown in Figure 3, the mRNA levels of MMP-2, MMP-9, and u-PA were significantly reduced after different treatments compared to that in the control group (all P<0.01). The mRNA levels of MMP-2, MMP-9, and u-PA were significantly lower in the combination group compared to those in the ultrasound group and the baicalein group. All the differences were all statistically significant (P<0.01).

Reduced expression of MMP-2, MMP-9, and u-PA proteins
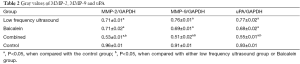
To further elucidate the mechanism underlying the effect of different treatments on the invasive activity of MDA-MB-231 cells, expression of MMP-2, MMP-9, and u-PA at protein level, before and after the treatments, was analyzed by western blotting. The results showed that the protein levels of MMP-2, MMP-9 and u-PA were significantly reduced after different treatments compared to those in the control group (P<0.05). The protein levels of MMP-2, MMP-9, and u-PA were significantly lower in the combination group compared to those in the ultrasound group and the baicalein group. All the differences were all statistically significant (P<0.05) (Figure 4, Table 2).
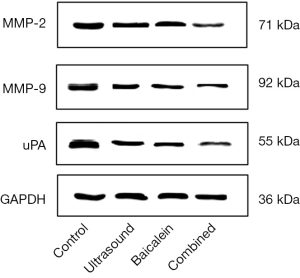
Full table
Discussion
Tumor metastasis is defined as the process of primary tumors invading and metastasizing to the surrounding or distal tissues and organs. It was reported that tumor cells can pass through the extracellular matrix via epithelial mesenchymal cell transformation (EMT) (11). In addition, tumor cells can secrete soluble proteolytic enzymes such as MMPs and u-PA to degrade the extracellular matrix, leading to the destruction of the basement membrane and metastasis to distant tissues and organs (12).
Baicalein is one of the important components in a Chinese herbal medicine, skullcaps. It has multiple pharmacological effects, including immune function regulation, anti-inflammation, and helps in reducing blood lipids (13). Recent reports in the literature showed that baicalein can inhibit cell proliferation, invasion and metastasis, and regulate signal transduction in several solid tumors, including breast cancer (14). Previous studies have demonstrated that tumor cells may be more susceptible to ultrasound radiation, when compared to normal cells, and that ultrasound can kill tumor cells by cavitation effect (15,16). Low frequency ultrasound presents several advantages, including fewer adverse biological effects and reduced drug resistance (17). In our study, we demonstrated, by using transwell migration assay, that baicalein and low frequency ultrasound treatment can reduce the invasive capacity of MDA-MB-231 breast cancer cells, which was consistent with previous studies (15-17). Furthermore, our study found that the combined use of the two treatments had stronger inhibitory effect on the invasive capacity of breast cancer cells compared to either one of the treatments individually, indicating the synergistic effect of the two treatments. To our knowledge, this is the first study to report the combined use of low frequency ultrasound and baicalein in the treatment of breast cancer, and revealed the synergistic effect of the two treatments.
In order to further explore the potential molecular underlying mechanism, the expression levels of tumor invasion associated molecules, such as MMPs and u-PA, were examined at both the transcriptional and translational levels. MMPs are widely expressed in tumor cells, and participate in a variety of tumor-specific behaviors, including invasion and metastasis (12). MMPs can degrade collagen fibers, thereby enabling the tumor cells to migrate into the surrounding environment. Accumulating evidence indicates that MMPs, specifically MMP-2 and MMP-9, are associated with the invasion and metastasis in breast cancer (18). u-PA is a type of protease, that can degrade the basement membrane components around the tumor tissue by proteolytic hydrolysis. It was demonstrated that downregulation of u-PA can inhibit the invasive capacity of BT-549 breast cancer cells (19). The results of this study showed that the expression levels of MMP-2, MMP-9, and u-PA were downregulated at both the protein and mRNA levels after treatment with either low frequency ultrasound or baicalein. The results of our study also indicated that low frequency ultrasound and baicalein may inhibit tumor cell invasion by downregulating the expression of MMP-2, MMP-9, and u-PA. We further examined the changes in the expression of these genes in the combination group, and the results showed that the reduction in the expression of MMP-2, MMP-9, and u-PA at both the transcriptional and translational levels was significantly higher in the combination group compared to that with either treatment alone. These results further confirmed the synergistic effect of the two treatments, and indicated that the combined use of low frequency ultrasound and baicalein may be the optimal strategy for breast cancer therapy.
This study has some limitations. Firstly, only a few of the tumor invasion associated molecules were examined in this study, since this was a preliminary study. Secondly, the effects of MMPs and u-PA on the invasion of breast cancer cells were not further validated by reinstating the expression of these molecules. Thirdly, it is necessary to further confirm these findings in vivo by using tumor bearing mice or other animal models. Further research is needed to explore the exact mechanism underlying the inhibitory effects of low frequency ultrasound and baicalein on breast cancer invasion and their synergistic effects in vitro and in vivo.
In summary, our study results indicated that the combined use of low frequency ultrasound and baicalein was a novel and optimized strategy for breast cancer therapy, which resulted in better inhibitory effect on the invasion of MDA-MB-231 breast cancer cells, probably by downregulating the expression of MMP-2, MMP-9, and u-PA. The findings of this study will be helpful in the identification of potential targets for targeted treatment of breast cancer, and further development of this novel therapeutic strategy.
Acknowledgments
Funding: This work was partially supported by the National Natural Science Foundation of China (Grant No. 81301200, 81201069).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.12.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Huang ZZ, Chen WQ, Wu XC, et al. Incidence and mortality of female breast cancer in China-a report from 32 Chinese cancer registries, 2003-2007. Tumor 2012;32:435-9.
- Li N, Zheng RS, Zhang SW, et al. Analysis and prediction of breast cancer incidence trend in China. Zhonghua Yu Fang Yi Xue Za Zhi 2012;46:703-7. [PubMed]
- Chia KS, Reilly M, Tan CS, et al. Profound changes in breast cancer incidence may reflect changes into a Westernized lifestyle: a comparative population-based study in Singapore and Sweden. Int J Cancer 2005;113:302-6. [Crossref] [PubMed]
- McPherson K, Steel CM, Dixon JM. ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. BMJ 2000;321:624-8. [Crossref] [PubMed]
- Marx V. Tracking metastasis and tricking cancer. Nature 2013;494:133-6. [Crossref] [PubMed]
- Wang YC, Wei LJ, Liu JT, et al. Comparison of Cancer Incidence between China and the USA. Cancer Biol Med 2012;9:128-32. [PubMed]
- Linos E, Spanos D, Rosner BA, et al. Effects of reproductive and demographic changes on breast cancer incidence in China: a modeling analysis. J Natl Cancer Inst 2008;100:1352-60. [Crossref] [PubMed]
- Lejbkowicz F, Salzberg S. Distinct sensitivity of normal and malignant cells to ultrasound in vitro. Environ Health Perspect 1997;105:1575-8. [Crossref] [PubMed]
- Mao J, Xu SS, Shen LL, et al. Anti-tumor effect and its mechanism of baicalein. Chinese Journal of Clinical Pharmacology and Therapeutics 2009;14:1178-82.
- Lamouille S, Subramanyam D, Blelloch R, et al. Regulation of epithelial-mesenchymal and mesenchymal-epithelial transitions by microRNAs. Curr Opin Cell Biol 2013;25:200-7. [Crossref] [PubMed]
- Liu KC, Huang AC, Wu PP, et al. Gallic acid suppresses the migration and invasion of PC-3 human prostate cancer cells via inhibition of matrix metalloproteinase-2 and -9 signaling pathways. Oncol Rep 2011;26:177-84. [PubMed]
- Wu WJ, Ding QL. Research progress of pharmacology of baicalein. Jiangsu Pharmaceutical and Clinical Research 2006;14:103-7.
- Günther S, Ruhe C, Derikito MG, et al. Polyphenols prevent cell shedding from mouse mammary cancer spheroids and inhibit cancer cell invasion in confrontation cultures derived from embryonic stem cells. Cancer Lett 2007;250:25-35. [Crossref] [PubMed]
- Lecornet E, Ahmed HU, Moore C, et al. Focal therapy for prostate cancer: a potential strategy to address the problem of overtreatment. Arch Esp Urol 2010;63:845-52. [PubMed]
- Lejbkowicz F, Zwiran M, Salzberg S. The response of normal and malignant cells to ultrasound in vitro. Ultrasound Med Biol 1993;19:75-82. [Crossref] [PubMed]
- Bai W, Shen E, Hu B. Research progression of low-frequency ultrasound and microbubble contrast agent in tumor therapy. Chinese Journal of Clinicians 2011;5:115-7. (Electronic Edition).
- Wang XJ, Zhou SF, Shi WF, et al. Relationship between the expression of uPA and MMP-9 in invasive ductal carcinoma of breast. Sichuan Medical Journal 2009;30:1200-2.
- Huang S, New L, Pan Z, et al. Urokinase plasminogen activator/urokinase-specific surface receptor expression and matrix invasion by breast cancer cells requires constitutive p38alpha mitogen-activated protein kinase activity. J Biol Chem 2000;275:12266-72. [Crossref] [PubMed]


