
Hypoxia-induced DNA hypermethylation: another reason to normalize tumor vessels
As solid tumors grow, regions within the tumors often experience a deficit in oxygen, known as hypoxia. Hypoxia results from an imbalance in oxygen supply and demand. There is an increase in oxygen demand as cells proliferate or get recruited to build the tumor biomass, while oxygen supply becomes limited as the blood vessels within the growing tumor are absent and/or become abnormal in their structure and function (1,2). In addition to causing cell death, hypoxia can also promote tumor progression, immunosuppression, and treatment resistance. Indeed, tumor hypoxia is associated with poor patient outcome (3). This somewhat counter-intuitive relationship is, in part, due to the ability of tumors to adapt to and evolve in oxygen- and nutrient-deficient microenvironments. A recent report by Thienpont et al. demonstrated that one such mechanism may be through epigenetic regulation of gene expression by the ten-eleven translocation (TET) methylcytosine dioxygenases (4). This exciting finding provides another rationale to normalize the tumor vasculature to alleviate hypoxia and its consequences.
The TET enzymes are a family of oxygen-, α-ketoglutarate- and iron (Fe2+)-dependent proteins that mediate DNA demethylation by catalyzing the oxidation of 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC). Aberrant alterations in DNA methylation, in particular the hypermethylation of CpG promoter islands, result in downregulation of tumor suppressor genes, and have been well documented in a variety of cancers (5). Reduced levels of 5hmC have also been found in many cancers (6). Thienpont and colleagues recently showed that such 5hmC loss and DNA hypermethylation in tumors is mediated by oxygen-dependent TET activity and have a microenvironmental cause—hypoxia (4). Through in vitro experiments, high throughput DNA immunoprecipitation sequencing (DIP-seq), and RNA sequencing of hypoxic cells, they demonstrated that hypoxia causes a reduction of TET enzyme activity and 5hmC in many cell lines, resulting in DNA promoter hypermethylation and repression of gene expression. A series of bioinformatic analyses of data from The Cancer Genome Atlas (TCGA) as well as experiments with genetically engineered mouse models of breast cancer further supported this link between hypoxia, reduced TET activity, and DNA hypermethylation (4). These data indicated that hypoxia could be associated with up to 48% of hypermethylation events in solid tumors. Moreover, hypoxia-induced reduction in TET activity seemed to result directly from the decrease in oxygen availability and not due to cell proliferation or changes in availabilities of the other TET cofactors (α-ketoglutarate, Fe2+ and vitamin C). Supplementation with cell permeable α-ketoglutarate or ascorbate did not rescue the effect of hypoxia on 5hmC loss. Importantly, the hypoxia-induced increase in DNA hypermethylation occurred in the promoters of tumor suppressor genes but not of oncogenes, suggesting an association with tumor progression. These results underscore the importance of molecular oxygen as a critical factor in the cellular biochemical processes involved in oncogenesis.
DNA hypermethylation now joins a myriad of other mechanisms by which hypoxia can alter gene and protein expression in favor of tumor survival and progression (Figure 1). Hypoxia is already well known to regulate gene expression through the stabilization of hypoxia-inducible factor (HIF) α-subunits, which results in downstream transcription of hundred of genes, including those involved in cell cycle progression, apoptosis, angiogenesis, anaerobic metabolism, metastasis, stem cell-like phenotype, fibrosis, DNA repair and immune evasion [recently reviewed in (7-10)]. Independent of HIF, hypoxia also inhibits signaling downstream of the mammalian target of rapamycin (mTOR) pathway, as well as triggers the unfolded protein response (UPR) pathway (11). Since the reduction of TET activity in response to hypoxia appeared to be independent of HIF pathway activation (4), it would be interesting to see if and how DNA hypermethylation due to reduced TET activity crosstalks with the other hypoxia response pathways in tumors. For example, are the genes that become repressed after hypoxia-induced 5hmC loss also part of the HIFα, mTOR or UPR signaling pathways? Thienpont and colleagues have reported that the hypermethylated genes in hypoxic tumors are associated with cell cycle arrest, DNA repair, apoptosis, glycolysis, angiogenesis and metastasis (4), similar to those downstream of HIF signaling. TET inhibition has also been previously shown to down-regulate the expression levels of some HIF target genes (12). Given the extensive genetic, epigenetic and metabolic reprogramming in response to hypoxia, and the crosstalk among the molecular players involved in key cellular processes, it is possible for hypoxia-induced DNA hypermethylation to act both in concert with and as checks-and-balances against other hypoxia response pathways.
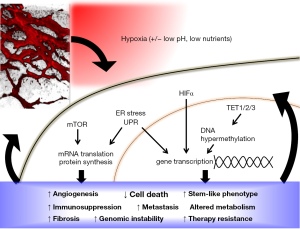
Characterizing how hypoxia-induced DNA hypermethylation integrates with other oncogenetic and hypoxia response pathways will also help clarify its functional significance in tumors. So far, it remains unclear if and how the hypermethylation of tumor suppressor genes in response to hypoxia contributes to tumor aggressiveness. In the MMTV-PyMT mouse models of breast cancer used by Thienpont and colleagues, hypoxia-induced 5hmC loss and DNA hypermethylation did not seem to affect tumor growth, perhaps because the tumor growth was strongly driven by the PyMT oncogenic transgene. Reports by other groups have actually shown that TET1 and TET3 protein expression levels are positively associated with tumor malignancy and poor prognosis, and can be upregulated by hypoxia in a HIF1α-dependent manner [reviewed in (13)]. Indeed, four out of the fifteen cell lines used by Thienpont and colleagues showed an increase in TET expression via HIF-mediated transcriptional upregulation after hypoxia, resulting in no change or even increase in 5hmC levels, in contrast to the general trend observed in the other eleven cell lines and in tumor models. Furthermore, TET activity may be affected by factors other than hypoxia. In a small subset of solid tumors, TET expression and activity are inhibited by genetic mutations, such as isocitrate dehydrogenase (IDH) gain-of-function mutations (14,15), mutations in succinate dehydrogenase and fumarate hydratase (12), as well as loss-of-function mutations in the TET proteins themselves (16,17). TET activity is also regulated by the availability of its metabolite cofactors, which are in turn regulated by changes in cellular metabolism in response to hypoxia, oxidative stress and lack of nutrients (8,18). The effects of hypoxia on DNA hypermethylation and repression of downstream gene expression, and their subsequent effect on oncogenesis, is therefore likely to be highly dependent on the cell- and tumor-type as well as the local microenvironment. Of note, cancer cells constitute only one of many cell types in a solid tumor. Tumor stromal cells such as cancer-associated fibroblasts, mesenchymal stem cells, immune cells and endothelial cells all experience and respond to hypoxia. The role of hypoxia in regulating TET activity and DNA methylation status in the tumor stromal cells, and how it may contribute to tumor development, remains to be determined.
Further complicating this landscape is the fact that hypoxia in tumors can be temporally and spatially heterogeneous. The tumor vasculature is leaky and disorganized. Some regions within the tumor may be devoid of functional blood vessels, resulting in chronic hypoxia, while other regions may experience acute hypoxia as blood flow through the tortuous tumor vessels becomes transiently blocked or reversed or deoxygenated (3,19). The severity of hypoxia also varies intra- and inter-tumorally, with both chronic and acute hypoxia triggering a host of different cellular adaptation pathways (3,20). Similarly, the level of available oxygen can modulate cellular hypoxia response in a dose-dependent manner. For example, the activity of the TET enzymes is only reduced at oxygen levels at or below 2%, but not at mild hypoxic levels (2–5% O2) (4,12). Moreover, the abnormal tumor vasculature also compromises the delivery of nutrients, which places additional stress on cells and limits the availability of metabolites that can affect tumor response to hypoxia (8). As 5hmC modification is dynamic and reversible, it remains to be seen how the distribution of DNA hypermethylation events changes in accordance to tumor hypoxia over time, and how the changes promote tumor progression.
Given the effects of hypoxia on tumors, alleviating hypoxia and countering the effects of hypoxia are attractive strategies for slowing tumor progression and improving cancer treatment (7). The discovery that TET activity loss mediates hypoxia-induced tumor DNA hypermethylation makes the TET enzymes and their associated pathway molecules possible drug targets for countering the effects of hypoxia (14,17,21). Nevertheless, the dysfunctional tumor vessels that cause hypoxia also lead to poor drug delivery into tumors, resulting in treatment resistance (2). Furthermore, oxygen plays an important role in response to multiple cancer therapies including radiotherapy, chemotherapy and immunotherapy (22). Thus, alleviating hypoxia by targeting its root cause—the abnormal tumor vasculature, represents a rational approach for improving the treatment outcome. Strategies to normalize the tumor vasculature include the use of anti-angiogenic agents at a judicious dosage and schedule, which helps the maturation rather than the pruning of blood vessels (2). Treatment with high doses and/or long periods of anti-angiogenic therapies inhibits blood vessel formation and actually creates more hypoxia, which may impair therapeutic outcome (7). Because solid tumors often have a high density of cancer cells and/or stromal components such as cancer associated fibroblasts and extracellular matrix proteins that can physically compress blood vessels (23), vascular normalization may also involve the use of anti-desmoplastic agents to reduce the mechanical stress within tumors (2). For example, antagonists of the renin-angiotensin system such as angiotensin receptor blockers have been shown to decrease the level of collagen I and hyaluronan in tumors, allowing for compressed blood vessels to open up and become perfused again in mouse models of breast and pancreatic cancer (24). There is a growing body of evidence that the use of anti-angiogenic and/or anti-desmoplastic agents in combination with cytotoxic anti-tumor chemotherapy and immunotherapy can produce beneficial outcome for cancer patients in both preclinical and clinical models (7).
Ultimately, the dynamic heterogeneity of solid tumors necessitates a personalized approach towards treating cancer. Biomarkers that predict patient outcome and response to therapy are useful for the rational stratification of patients and the design of treatment strategies. In terms of treatments to alleviate tumor hypoxia, clinical studies have revealed that the extent of vascular normalization and tissue oxygenation after anti-angiogenic therapy correlate with greater progression-free survival and overall survival in both newly diagnosed and recurrent glioblastoma patients (7). Measuring hypoxia levels in tumors with oxygen-sensitive probes (25) or with surrogate markers such as blood perfusion via MRI (26) may help identify patients who are likely to benefit from treatment. Perhaps TET activity and DNA hypermethylation status in tumors or circulating DNA may also be used as prognostic markers to determine the extent of hypoxia and the risk of tumor progression.
The complexity that underlies the effects of hypoxia on cancer development and treatment resistance highlights the need for a greater understanding of solid tumors at the systems-level. Especially with the recent success of immunotherapies for cancer, investigations should not only continue to dissect the molecular details of the hallmarks of cancer, but also seek to integrate information from the network of signaling pathways that occurs at the cellular level, to the whole tumor level where tumor cells interact with a host of stromal cells, extracellular matrices and secreted soluble factors, and finally to the whole body level where the vasculature, the nervous system and the immune system all intersect and play a role. The development of experimental tools and bioinformatics analyses methods for “-omic” level (i.e., genomics, proteomics, epigenomics and metabolomics) research has increased our understanding of the systems biology of cancer. A holistic and multi-dimensional understanding of interactions between cancer cells and their microenvironment will aid in finding new ways to inhibit tumor progression and therapeutic resistance.
Acknowledgments
Funding: MR Ng was supported by a US Department of Defense Breast Cancer Research Program Postdoctoral Fellowship (W81XWH-14-1-0034). RK Jain was supported by the Program Project Grant (P01-CA080124) and Outstanding Investigator Award (R35-CA197743) from the NIH National Cancer Institute.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Chen Qian (Center for Inflammation & Epigenetics, Houston Methodist Hospital Research Institute, Houston, TX, USA).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.12.72). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 2005;307:58-62. [Crossref] [PubMed]
- Martin JD, Fukumura D, Duda DG, et al. Reengineering the Tumor Microenvironment to Alleviate Hypoxia and Overcome Cancer Heterogeneity. Cold Spring Harb Perspect Med 2016;6.
- Vaupel P, Mayer A. Hypoxia in tumors: pathogenesisrelated classification, characterization of hypoxia subtypes, and associated biological and clinical implications. In: Swartz HM, Harrison DK, Bruley DF. editors. Oxygen Transport to Tissue XXXVI. New York, NY: Springer New York, 2014:19-24.
- Thienpont B, Steinbacher J, Zhao H, et al. Tumour hypoxia causes DNA hypermethylation by reducing TET activity. Nature 2016;537:63-8. [Crossref] [PubMed]
- Rodríguez-Paredes M, Esteller M. Cancer epigenetics reaches mainstream oncology. Nat Med 2011;17:330-9. [Crossref] [PubMed]
- Pfeifer GP, Kadam S, Jin SG. 5-hydroxymethylcytosine and its potential roles in development and cancer. Epigenetics Chromatin 2013;6:10. [Crossref] [PubMed]
- Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 2014;26:605-22. [Crossref] [PubMed]
- Nakazawa MS, Keith B, Simon MC. Oxygen availability and metabolic adaptations. Nat Rev Cancer 2016;16:663-73. [Crossref] [PubMed]
- Ratcliffe PJ. Oxygen sensing and hypoxia signalling pathways in animals: the implications of physiology for cancer. J Physiol 2013;591:2027-42. [Crossref] [PubMed]
- Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol 2014;9:47-71. [Crossref] [PubMed]
- Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer 2008;8:851-64. [Crossref] [PubMed]
- Laukka T, Mariani CJ, Ihantola T, et al. Fumarate and Succinate Regulate Expression of Hypoxia-inducible Genes via TET Enzymes. J Biol Chem 2016;291:4256-65. [Crossref] [PubMed]
- Chen HF, Wu KJ. Epigenetics, TET proteins, and hypoxia in epithelial-mesenchymal transition and tumorigenesis. Biomedicine (Taipei) 2016;6:1. [Crossref] [PubMed]
- Cairns RA, Mak TW. Oncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunities. Cancer Discov 2013;3:730-41. [Crossref] [PubMed]
- Flavahan WA, Drier Y, Liau BB, et al. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature 2016;529:110-4. [Crossref] [PubMed]
- Nickerson ML, Das S, Im KM, et al. TET2 binds the androgen receptor and loss is associated with prostate cancer. Oncogene 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Thienpont B, Galle E, Lambrechts D. TET enzymes as oxygen-dependent tumor suppressors: exciting new avenues for cancer management. Epigenomics 2016;8:1445-8. [Crossref] [PubMed]
- Kinnaird A, Zhao S, Wellen KE, et al. Metabolic control of epigenetics in cancer. Nat Rev Cancer 2016;16:694-707. [Crossref] [PubMed]
- Helmlinger G, Yuan F, Dellian M, et al. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med 1997;3:177-82. [Crossref] [PubMed]
- Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer 2008;8:180-92. [Crossref] [PubMed]
- Jones PA, Issa JP, Baylin S. Targeting the cancer epigenome for therapy. Nat Rev Genet 2016;17:630-41. [Crossref] [PubMed]
- Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer 2011;11:393-410. [Crossref] [PubMed]
- Stylianopoulos T, Martin JD, Chauhan VP, et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc Natl Acad Sci U S A 2012;109:15101-8. [Crossref] [PubMed]
- Chauhan VP, Martin JD, Liu H, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun 2013;4:2516. [Crossref] [PubMed]
- Roussakis E, Li Z, Nichols AJ, et al. Oxygen-Sensing Methods in Biomedicine from the Macroscale to the Microscale. Angew Chem Int Ed Engl 2015;54:8340-62. [Crossref] [PubMed]
- Emblem KE, Mouridsen K, Bjornerud A, et al. Vessel architectural imaging identifies cancer patient responders to anti-angiogenic therapy. Nat Med 2013;19:1178-83. [Crossref] [PubMed]


