Hypofractionated radiotherapy for breast cancer: how to evaluate chronic toxicity and cosmesis?
Background
Adjuvant irradiation constitutes an important component in the postoperative treatment of breast cancer, reaching as far as 25–30% of workload in radiotherapy departments. Some approaches to reduce delays and optimize available resources include accelerated schedules of daily doses slightly superior to standard ones (1). Several trials had utilized altered fractionations ranging from 2.5 Gy in 16 fractions to 3.3 Gy in 13 fractions (2,3), showing almost an equivalent safety and effectiveness compared to standard fractionation. These results have been confirmed in large randomized trials. In the Ontario Clinical Oncology Group published by Whelan et al. (4), 1,234 patients with T1-2N0 tumors were randomized to receive 42.5 Gy in 16 fractions compared with the standard 50 Gy in 25 fractions. After a follow-up of 10 years the local control was 93.8% and 93.3% in both groups respectively and the chronic toxicity and cosmesis was similar in both arms. In UK START A (5) and B (6) trials similar results were obtained confirming the same safety and efficacy of the hypofractionation schedule compared to the standard one, and also with a slightly less chronic toxicity and better cosmesis in the hypofractionated arms. According to these good results, daily hypofractionation is actually the preferred choice after conservative surgery in breast cancer, has been included in recommendations and clinical guidelines (7), and is considered the standard of care (8). In conclusion, moderate hypofractionation is a safe treatment modality without compromising effectiveness and has direct implications in health care systems (9).
Toxicity and cosmesis analysis
Implementation of hypofractionated schedules has been delayed because of concerns about late toxicity results and cosmesis at long-term follow-up. Report of toxicity results and cosmesis have been normally evaluated on basis on some physician collected data by validated scales, patient reported outcomes or both. Although some authors have reported long term effects at 10 years, there’s no consensus in methodology and evaluation tools employed and there is limited data about of evolution of chronic toxicity and cosmesis across time.
For this reason the study published by Swanick et al. (10) is an important contribution to confirm the results of hypofractionation compared to conventionally fractionated whole-breast irradiation. In this trial, patient were randomized to receive 50 Gy in 25 fractions or hypofractionation of 42.6 Gy in 16 fractions followed by a boost of 10 or 14 Gy in the standard dose arm and 10–12.5 Gy in 4–5 fractions of 2.5 Gy in the hypofractionated arm, depending on margin status. Several patient-rated evaluations were performed by means of Breast Cancer Treatment Outcome Scale (BCTOS), Functional assessment of Cancer therapy-Breast (FACT-B) and Body Image Scale, allowing an exhaustive evaluation of cosmetic status and body image related symptoms and distress, functional status, well-being and emotional impact of the whole treatment. Also a cosmesis rating by RTOG scale was assessed by the corresponding physician. All evaluations were performed at baseline, at 6 months and yearly at 1 year, 2 and 3 years after completion of irradiation. An amount of 287 patients with a median follow-up of 2 years were assessed (149 treated with conventional fractionation and 138 with hypofractionated irradiation). From the patient point of view the patient reported outcomes were similar and the BCTOS showed slight better results in the hypofractionated arm. Interestingly an improvement over time at clinical level (functional status, breast pain) was observed and was evident in both arms. Finally the physician reported scores on cosmesis showed no differences between arms and also at any of the scheduled time point of the follow-up. So, the importance of this work lies in the exhaustive outcome assessment and the continuous evaluation over time. As stated by the authors one of the limitations of this work is that all series has not reached already the planned follow-up of 3 years despite there was no impact of the effect of missing data.
New approaches in toxicity assessment
There are several attempts to obtain complementary information or alternatives to clinical scales or subjective evaluation to assess acute and chronic toxicity. Some authors have explored the utility of skin or subcutaneous change analysis in the evaluation of dermatitis by means skin probes or ultrasounds devices. In a work of González and colleagues (11), patients were prospectively assessed by real-time, laser doppler flow measurement before and after irradiation and compared to the non irradiated breast. The authors conclude that such objective testing permits more accurate toxicity intensity grading and that sometimes the findings are evident prior to the onset of clinical symptoms. Other studies suggest that similar devices could be useful to evaluate at long term both skin pigmentation changes or elasticity (12). In our experience we have explored the utility of a multiprobe skin tester (Multi-skin Center MC 750-B2) to analyze redness, hyperpigmentation, hydration and elasticity in cohorts of patients treated with diverse schedules of irradiation: standard fractionation, daily hypofractionation, weekly hypofractionation and hyperfractionation in patients included in a partial breast irradiation randomized trial (13). In 50 patients treated with hypofractionation of 40.5 Gy in 15 fractions with a boost, we found that the increased redness and the reduced elasticity correlated well to the chronic evaluations by RTOG scales at a median follow-up of 3 years (14).
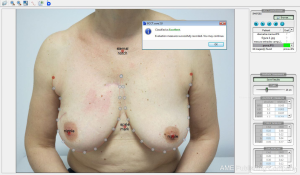
Some of the large randomized trials on hypofractionation have evaluated cosmesis results by adding photographic assessment prior and after breast irradiation. Evaluation was performed by visual comparison of a sequence of images attending to their morphologic changes according to size, symmetry, retraction or color impairments. More recently with the aid of computer assisted image evaluation tools all this process become more reliable, quicker, and can include an overall score of final cosmesis that can be compared to the physician-rated scales or the cosmesis evaluated by patients. The more extended one is the BCCT-Core software (15), showed in Figure 1, that has been utilized in the EORT boost trial 22881-10882 (16), the Young Boost Trial (17) and other important trials of intra-operative irradiation (TARGIT Trial) that will be published soon, as well as in our series of hypofractionated breast irradiation. We have found that the software scores correlate better with patient evaluation than physician-rated evaluation (correlation factor of 0.36 and 0.26 respectively; P=0.016).
The boost and their impact in the clinical outcomes after whole breast irradiation
Another issue of concern is the management of boost doses to tumor bed after whole breast hypofractionation. It is well known that the majority of local relapses present in 10–20 mm around the tumor and are more frequent in the first two years of follow-up. There is dose-response relationship in breast cancer (18); according to this, additional doses to the tumor bed improve local control and impacts on survival. In fact for every four local relapses prevented we can avoid a breast cancer death (19). The large randomized EORTC 22881-10882 trial (20) demonstrated that additional doses of 16 Gy prevents ipsilateral local relapse in 91% compared to 87% of patients without a boost at a follow-up of 20 years (hazard ratio 0.65; 95% CI, 0.52–0.81) and the advantage was more marked in young patients, high grade tumors and coexistence of intraductal carcinoma. So, an additional boost remains as a gold standard for the majority of patients after conservative surgery in breast cancer. The counterpart of the boost addition is some increase in chronic toxicity in boost area.
In trials investigating whole breast hypofractionation solely the published by Whelan et al. (4) didn’t applied a tumor boost. In the START trials the use of a boost was left at discretion of the investigator (5,6). However in those trials, when performed, the boost was administered at a standard fractionation up to 10 or 16 Gy. More recently several studies in patients treated with hypofractionation doses between 2.65 to 3.4 Gy have administered additional hypofractionated boost with marked variability. In these cases doses ranging from 2.5 to 8 Gy with photons or single dose with intra-operative electrons up to 12 Gy were applied. With the advent of the new technologies with advances such intensity modulated irradiation, or prone immobilization devices we can go one step forward in the integration of boost simultaneously to standard or hypofractionated irradiation, the so called concomitant boost (21). The concurrent doses to tumor bed are usually around 2.3 Gy in patients treated with standard fractionation to the whole breast and range from 3.2 to 3.53 Gy in the main ongoing trials of hypofractionation with concomitant boost as RTOG 1005 (22), the UK IMPORT-HIGH (23) or the UBZ Trial (24).
One step ahead in the use of accelerated hypofractionation is represented by the UK-FAST-Forward trial. In this study the patients were randomized to receive 40 Gy in 15 fractions of 2.67 Gy compared to a more compressed hypofractionation of 27 or 26 Gy in 5 consecutive doses of 5.4 or 5.2 Gy administered in one week (25). When indicated, in approximately 15% of cases, the boost was administered by standard fractions up to 10 or 16 Gy. The critique to this scenario is that the boost lasts the same time or more than the whole breast irradiation, losing the advantages of a shortened treatment in terms of patient convenience, tolerance and treatment unit workload.
In our personal experience we have administered the boost even at the same doses of 2.67 Gy up to 8 in 3 fractions (low boost) or 16 Gy in 6 fractions (high boost) after a total dose of 40.5 Gy to the whole breast. The indication of the dose of the boost was decided according to the cumulative presence of risk factor for local relapse including age, tumor, size, grade, intraductal carcinoma presence or margin status. The choice of total dose was applied after undertaken equivalence comparison from the radiobiological point of view as showed in Table 1. Interestingly our schedule of 15 doses to whole breast and additional boost in 6 fractions compares quite well with the classical 50 Gy plus a 16 Gy boost in term of biological equivalent dose to tumor and also for chronic toxicity in normal tissues (14).
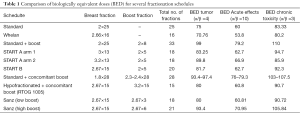
Full table
In conclusion, considering that hypofractionation is nowadays the standard of care in the majority of patients after breast conservative surgery, it is imperative an exhaustive and longitudinal evaluation of clinical results in terms of chronic toxicity and cosmesis results particularly when a boost is included after whole breast irradiation.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor San-Gang Wu (Department of Radiation Oncology, Xiamen Cancer Center, the First Affiliated Hospital of Xiamen University, Xiamen, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.12.58). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fisher CM, Rabinovitch R. Frontiers in radiotherapy for early-stage invasive breast cancer. J Clin Oncol 2014;32:2894-901. [Crossref] [PubMed]
- Ashworth A, Kong W, Whelan T, et al. A population-based study of the fractionation of postlumpectomy breast radiation therapy. Int J Radiat Oncol Biol Phys 2013;86:51-7. [Crossref] [PubMed]
- Wang EH, Mougalian SS, Soulos PR, et al. Adoption of hypofractionated whole-breast irradiation for early-stage breast cancer: a National Cancer Data Base analysis. Int J Radiat Oncol Biol Phys 2014;90:993-1000. [Crossref] [PubMed]
- Whelan T, MacKenzie R, Julian J, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst 2002;94:1143-50. [Crossref] [PubMed]
- START Trialists' Group. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol 2008;9:331-41. [Crossref] [PubMed]
- START Trialists' Group. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet 2008;371:1098-107. [Crossref] [PubMed]
- Smith BD, Bentzen SM, Correa CR, et al. Fractionation for whole breast irradiation: an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;81:59-68. [Crossref] [PubMed]
- Harnett A. Fewer fractions of adjuvant external beam radiotherapy for early breast cancer are safe and effective and can now be the standard of care. Why the UK's NICE accepts fewer fractions as the standard of care for adjuvant radiotherapy in early breast cancer. Breast 2010;19:159-62. [Crossref] [PubMed]
- Montero A, Sanz X, Hernanz R, et al. Accelerated hypofractionated breast radiotherapy: FAQs (frequently asked questions) and facts. Breast 2014;23:299-309. [Crossref] [PubMed]
- Swanick CW, Lei X, Shaitelman SF, et al. Longitudinal analysis of patient-reported outcomes and cosmesis in a randomized trial of conventionally fractionated versus hypofractionated whole-breast irradiation. Cancer 2016;122:2886-94. [Crossref] [PubMed]
- González-Sanchís A, Vicedo-González A, Brualla-González L, et al. Looking for complementary alternatives to CTCAE for skin toxicity in radiotherapy: quantitative determinations. Clin Transl Oncol 2014;16:892-7. [Crossref] [PubMed]
- Crook J, Ludgate C, Malone S, et al. Final report of multicenter Canadian Phase III randomized trial of 3 versus 8 months of neoadjuvant androgen deprivation therapy before conventional-dose radiotherapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2009;73:327-33. [Crossref] [PubMed]
- Rodríguez N, Sanz X, Dengra J, et al. Five-year outcomes, cosmesis, and toxicity with 3-dimensional conformal external beam radiation therapy to deliver accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys 2013;87:1051-7. [Crossref] [PubMed]
- Sanz J, Rodríguez N, Foro P, et al. Hypofractionated boost after whole breast irradiation in breast carcinoma: chronic toxicity results and cosmesis. Clin Transl Oncol 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Cardoso JS, Cardoso MJ. Towards an intelligent medical system for the aesthetic evaluation of breast cancer conservative treatment. Artif Intell Med 2007;40:115-26. [Crossref] [PubMed]
- Immink JM, Putter H, Bartelink H, et al. Long-term cosmetic changes after breast-conserving treatment of patients with stage I-II breast cancer and included in the EORTC 'boost versus no boost' trial. Ann Oncol 2012;23:2591-8. [Crossref] [PubMed]
- Brouwers PJ, van Werkhoven E, Bartelink H, et al. Factors associated with patient-reported cosmetic outcome in the Young Boost Breast Trial. Radiother Oncol 2016;120:107-13. [Crossref] [PubMed]
- Denham JW. The radiation dose-response relationship for control of primary breast cancer. Radiother Oncol 1986;7:107-23. [Crossref] [PubMed]
- de Bock GH, Putter H, Bonnema J, et al. The impact of loco-regional recurrences on metastatic progression in early-stage breast cancer: a multistate model. Breast Cancer Res Treat 2009;117:401-8. [Crossref] [PubMed]
- Bartelink H, Maingon P, Poortmans P, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol 2015;16:47-56. [Crossref] [PubMed]
- Franco P, Cante D, Sciacero P, et al. Tumor bed boost integration during whole breast radiotherapy: a review of the current evidence. Breast Care (Basel) 2015;10:44-9. [Crossref] [PubMed]
- RTOG 1005 protocol information minimize: a phase iii trial of accelerated whole breast irradiation with hypofractionation plus concurrent boost versus standard whole breast irradiation plus sequential boost for early-stage breast cancer. Available online: https://www.rtog.org/clinicaltrials/protocoltable/studydetails.aspx?study=1005
- Import high: randomised trial testing dose escalated, intensity-modulated radiotherapy for women treated by breast conservation surgery and appropriate systemic therapy for early breast cancer. Available online: http://www.icr.ac.uk/our-research/our-research-centres/clinical-trials-and-statistics-unit/clinical-trials/import_high
- Van Parijs H, Miedema G, Vinh-Hung V, et al. Short course radiotherapy with simultaneous integrated boost for stage I-II breast cancer, early toxicities of a randomized clinical trial. Radiat Oncol 2012;7:80. [Crossref] [PubMed]
- Brunt AM, Wheatley D, Yarnold J, et al. Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3-week regimen delivered in the UK FAST-Forward Trial. Radiother Oncol 2016;120:114-8. [Crossref] [PubMed]