
[18F]-choline PET/CT as an imaging biomarker for primary liver cancers
Choline-based radiotracers were initially developed for prostate cancer imaging (1-4). Comparing to C-11 labeled native choline, the transport efficiency of F-18 labeled fluorinated choline (fluorocholine) is similar, and the substrate specificity of choline kinase, a key enzyme responsible for choline uptake, is also similar towards both for prostate cancer imaging (5). Fluorocholine is labeled with F-18 with a half-life of almost 2 hours for radio-decay, which is long enough for commercial production and dispensing. In addition, fluorocholine with a known dosimetry has comparable safety profile to that of the native choline (6).
Besides the known applications to prostate cancer imaging, the choline tracers have been “re-purposed” for PET imaging of primary liver cancers such as the hepatocellular carcinoma (HCC), the 3rd leading cause of cancer death worldwide with increasing incidence and mortality in the United States (7). A unique clinical study was published recently by Kwee et al., correlating fluorocholine PET imaging data with phospholipid profiling (8). This study is different from most previous published clinical studies on the same topic (9-11). Many of the previous studies are comparative in nature, between choline or fluorocholine and the commonly used FDG for PET imaging of HCC with histological correlation in some of these studies (12). What is missing in all these previous clinical investigations are cellular characterization and validation. Preclinical studies with a clinically relevant animal model of naturally occurring HCC indicated that the uptake of radio-labeled choline tracers retained in HCC seen on PET images reflects the augmented biosynthesis of phospholipids, mainly phosphatidylcholine (PC) (13-15). This recent clinical study validated the preclinical findings. In addition, the recent study is distinctive in several aspects as discussed in the following.
Applying principle component analysis
Patients whose tissue samples would become available afterwards were selected for the PET/CT scan with fluorocholine in the recent study by Kwee et al. (8). Tumor and adjacent liver tissues were profiled by liquid chromatography mass spectrometry, and different molecular species of PC quantified by mass-to-charge ratio. Furthermore, profiles of HCC phospholipids were projected onto two orthogonal principal component factors (PCF1 and PCF2) accounting for 80% of total profile variation. From this transformed viewpoint, it became more clear than ever that the mechanism of enhanced tumor uptake of fluorocholine is due to its metabolic fate: integration into the synthesis of a specific molecular species of highly saturated PCs (PCF2 loading), which correlated with quantitative features such as the tumor-to-liver ratio, etc., calculated from the PET images while this is not the case in the surrounding liver tissues. This is actually not obvious by looking at the raw profiles and the image data. Previous preclinical studies hinted that the metabolism of radio-choline in HCC follows mainly the CDP-choline pathway that resulted in adding the saturated fatty acids in PC, while the metabolic fate of the same fluorocholine in the liver parenchymal follows PE-methylation pathway that resulted in getting the longer-chain poly-unsaturated fatty acids in PC (15). Therefore, as shown in Figure 1, not all PCs are the same!
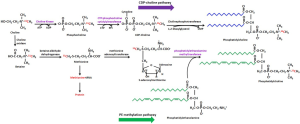
The recent clinical study went one step further. It showed through principle component projection that in addition to the PCs with short-chain highly unsaturated fatty acids (from de novo fatty acid synthesis), there is a mix from the PCs with long-chain poly-unsaturated fatty acids (derived from essential fatty acids), which results in a characteristic two-group pattern in HCC. In the surrounding liver tissues, there is no such distinct pattern. Interestingly, the study showed that the projected data has no correlation with histology-evaluated tumor grade, which is not a real surprise. As we are moving into precision medicine, the traditional descriptive cytopathology (histology-based, which has been the “ground true” for reference) started to give way to the more quantitative and molecular characterization.
Biomarker vs. target probe
Back to prostate cancer imaging, radio-cholines are currently being replaced gradually by more prostate cancer-specific radiotracers such as those newly developed peptide ligands targeting prostate specific membrane antigen (PSMA) (16) or gastrin-releasing peptide receptor (GRPR, or bombesin receptor) (17). The over-expression of these surface markers in tumor cells allowed targeted probes such as those radio-labeled peptide ligands to become effective for prostate cancer imaging. Radio-cholines are pathway markers reflecting up-regulated CDP-choline pathway as discussed above. Due to the nature of a pathway marker, fluorocholine positively depicted one intrahepatic cholangiocarcinoma in PET scan due to up-regulated CDP-choline pathway in that tumor. Since there is no PSMA-like specific markers for HCC so far, the choline tracers are here to stay and perhaps can be further investigated and validated as an PET imaging biomarker for a range of applications discussed by Kwee et al., such as for measuring the phospholipid effects of SREBP-1 antagonists, or other lipid pathway inhibitors, in tumors, or any prognostic or predictive values. To be an imaging biomarker, a quick static PET scan with fluorocholine along with simpler quantification is certainly desirable for routine use in a clinical setting. The recent clinical study confirmed that compartmental modeling using the dynamically acquired PET imaging data and the resultant parameter (k1–k4) estimation added no further or better correlation with the profiling data [Table 4 in (8)]. That can be explained in part by the fact that lipid profiling performed in this recent study was based on endogenous lipids, not radio-metabolite analysis of the radiotracer with its transient or intermediate products. Therefore, a static PET scan using fluorocholine with region-based analysis (tumor-to-background ratio, etc.) will be sufficient for use as a PET imaging biomarker for HCC applications.
Tumor inhomogeneity is also discussed in this recent study, and we are constantly reminded that PET imaging does not have the microscopic spatial resolution. Nevertheless, the strength of clinical PET imaging is its ability to present a composite readout of the disease state by reconciling the underlying inhomogeneity at multiple scales from genome to tissue. Furthermore, the quantitative nature of PET imaging can be an additional utility to the physicians for their decision-making in daily clinical practice.
Limitations
The drawback of almost all lipid-based small molecule radiotracers such as acetate and choline tracers for oncological applications is their uptake in pre-cancerous lesions (9,11) as up-regulation of the CDP-choline pathway for lipogenesis might have started with the pre-lesions. The recent study did not enroll patients with precancerous hepatic nodules, nor patients with advanced non-resectable liver tumors. Fortunately, PET scans with the choline tracers will not be used to differentiate pre-malignant from malignant lesions in most cases. The reason is that comparing to MRI or CT imaging, PET imaging is not a first line imaging modality or screening tool; rather, it is a problem solving technology when the results from other imaging modalities are equivocal. Patients would normally have their multi-phase contrast enhanced CT or MRI done before the PET scan was ordered. Regarding the advanced HCCs, anecdote results from preclinical studies showed that the imaging signal of choline uptake in these HCCs leveled down comparing to the early stage HCC, but still above liver background suggesting a possible re-partitioning the mixture between CDP-choline and PET methylation pathways in advanced HCCs for the metabolism of the choline tracers (14,15). This is worthwhile for further investigation both preclinically and clinically.
The main issue with choline tracers might still be the high liver background, which the data from the recent study indirectly addressed (8). No easy solution is in sight. Looking at the choline family, a study (18) showed that the transport system (choline is a charged molecule and needs to be actively transported into the cytosol) imposes a strict upper limit upon the size of substrate, which limits the structure of choline analogs. A small increase in the size of the quaternary ammonium head would sharply lower affinity for the transporter as large cholines failed to penetrate the cells. Intriguingly, another study (19) found that none of the choline analogs tested were better substrates than the simplest (native) choline for choline kinase with the exception of ethylcholine (Figure 2). A preclinical study confirmed this observation, in which the uptake of the native [11C]-chloine in HCC was comparable to that of [18F]-fluoroethylcholine (20). Choline analogs are not the answer to the issue of liver background, and new strategies will need to be developed.
Acknowledgments
Funding: The author is supported in part by NIH/NCI grant R01CA204373.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Long Chen (Department of PET-CT center at the Yunnan Tumor Hospital, The Third Affiliated Hospital of Kunming Medical University, Kunming, China; Department of Biochemistry and Molecular Biology of Kunming Medical University, Kunming, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.12.41). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- DeGrado TR, Coleman RE, Wang S, et al. Synthesis and evaluation of 18F-labeled choline as an oncologic tracer for positron emission tomography: initial findings in prostate cancer. Cancer Res 2001;61:110-7. [PubMed]
- Sutinen E, Nurmi M, Roivainen A, et al. Kinetics of [(11)C]choline uptake in prostate cancer: a PET study. Eur J Nucl Med Mol Imaging 2004;31:317-24. [Crossref] [PubMed]
- Schmid DT, John H, Zweifel R, et al. Fluorocholine PET/CT in patients with prostate cancer: initial experience. Radiology 2005;235:623-8. [Crossref] [PubMed]
- Reske SN, Blumstein NM, Neumaier B, et al. Imaging prostate cancer with 11C-choline PET/CT. J Nucl Med 2006;47:1249-54. [PubMed]
- Kwee SA, DeGrado TR, Talbot JN, et al. Cancer imaging with fluorine-18-labeled choline derivatives. Semin Nucl Med 2007;37:420-8. [Crossref] [PubMed]
- DeGrado TR, Reiman RE, Price DT, et al. Pharmacokinetics and radiation dosimetry of 18F-fluorocholine. J Nucl Med 2002;43:92-6. [PubMed]
- NIH. Action Plan for Liver Disease Research - A Report of the Liver Disease Subcommittee of the Digestive Diseases Interagency Coordinating Committee. 2004 Edition. National Institutes of Health, 2004.
- Kwee SA, Sato MM, Kuang Y, et al. [18F]Fluorocholine PET/CT Imaging of Liver Cancer: Radiopathologic Correlation with Tissue Phospholipid Profiling. Mol Imaging Biol 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Talbot JN, Gutman F, Fartoux L, et al. PET/CT in patients with hepatocellular carcinoma using [(18)F]fluorocholine: preliminary comparison with [(18)F]FDG PET/CT. Eur J Nucl Med Mol Imaging 2006;33:1285-9. [Crossref] [PubMed]
- Yamamoto Y, Nishiyama Y, Kameyama R, et al. Detection of hepatocellular carcinoma using 11C-choline PET: comparison with 18F-FDG PET. J Nucl Med 2008;49:1245-8. [Crossref] [PubMed]
- Talbot JN, Fartoux L, Balogova S, et al. Detection of hepatocellular carcinoma with PET/CT: a prospective comparison of 18F-fluorocholine and 18F-FDG in patients with cirrhosis or chronic liver disease. J Nucl Med 2010;51:1699-706. [Crossref] [PubMed]
- Kwee SA, Wong LL, Hernandez BY, et al. Chronic Liver Disease and the Detection of Hepatocellular Carcinoma by [(18)F]fluorocholine PET/CT. Diagnostics (Basel) 2015;5:189-99. [Crossref] [PubMed]
- Salem N, Kuang Y, Wang F, et al. PET imaging of hepatocellular carcinoma with 2-deoxy-2[18F]fluoro-D-glucose, 6-deoxy-6[18F] fluoro-D-glucose, [1-11C]-acetate and [N-methyl-11C]-choline. Q J Nucl Med Mol Imaging 2009;53:144-56. [PubMed]
- Kuang Y, Salem N, Corn DJ, et al. Transport and metabolism of radiolabeled choline in hepatocellular carcinoma. Mol Pharm 2010;7:2077-92. [Crossref] [PubMed]
- Kuang Y, Salem N, Tian H, et al. Imaging lipid synthesis in hepatocellular carcinoma with [methyl-11c]choline: correlation with in vivo metabolic studies. J Nucl Med 2011;52:98-106. [Crossref] [PubMed]
- Afshar-Oromieh A, Haberkorn U, Eder M, et al. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F-FECH. Eur J Nucl Med Mol Imaging 2012;39:1085-6. [Crossref] [PubMed]
- Kähkönen E, Jambor I, Kemppainen J, et al. In vivo imaging of prostate cancer using [68Ga]-labeled bombesin analog BAY86-7548. Clin Cancer Res 2013;19:5434-43. [Crossref] [PubMed]
- Devés R, Krupka RM. The binding and translocation steps in transport as related to substrate structure. A study of the choline carrier of erythrocytes. Biochim Biophys Acta 1979;557:469-85. [Crossref] [PubMed]
- Clary GL, Tsai CF, Guynn RW. Substrate specificity of choline kinase. Arch Biochem Biophys 1987;254:214-21. [Crossref] [PubMed]
- Kolthammer JA, Corn DJ, Tenley N, et al. PET imaging of hepatocellular carcinoma with 18F-fluoroethylcholine and 11C-choline. Eur J Nucl Med Mol Imaging 2011;38:1248-56. [Crossref] [PubMed]



