
DDR1 and Notch: a multifaceted synergy
In recent years our appreciation of the function and design of many signalling pathways implicated in cancer has changed substantially. The original idea of intrinsically independent and linear routes has progressively evolved to the concept of complex networks, involving extensive crosstalk and interplay between various signalling pathways. Yet, we are only beginning to understand how these networks are interconnected within cancer cells. This knowledge will be essential for the design of effective drug combinations as well as for the anticipation of potential mechanisms of resistance.
This burden of complexity was evident in our recent manuscript reporting an efficacious preclinical therapy based on the combined pharmacological inhibition of the receptor tyrosine kinase DDR1 and Notch signalling in K-Ras driven lung adenocarcinoma (1). Yet, as pointed out in the recent commentary by Dr. Lendahl, we are still far from understanding the underlying biology and the hierarchy that governs this K-Ras/DDR1/Notch interplay. Indeed, both positive and negative interactions between Notch and Ras have been identified in various tumour types where Notch can sustain or prevent tumour growth depending on the cellular context (2). We hypothesize that in K-Ras driven lung adenocarcinoma this context dependency may in part rely on the function of DDR1.
We identified DDR1 as the top hit in the transcriptional profiling of a subset of mouse lung hyperplasias when analysed shortly after the activation of a resident K-RasG12V oncogene. The limited consensus on a reliable Notch-dependent transcriptional profile in the published literature prevented us from analysing the presence of a Notch signature in these early lesions. Yet, in the early stages of pancreatic adenocarcinoma, oncogenic K-Ras fails to induce Notch target genes suggesting that these pathways converge subsequently during tumour initiation (3). Although we currently lack supporting experimental evidence, we can speculate on several non-mutually exclusive mechanisms that could explain this functional convergence.
In breast cancer cells, the interaction with DDR1 triggers Notch activation (4). We are still ignorant as to whether the same process operates in K-Ras-driven lung adenocarcinoma. If this is the case, and since DDR1 transcription is partly controlled by MAPK activity (5), K-Ras-induced DDR1 expression would precede Notch activation. Yet, it is currently unclear how DDR1 itself becomes activated. Collagens, the only known DDR1 ligands (5) are among the most abundant proteins in mammalian tissue suggesting that additional layers of regulation must exist to prevent unscheduled activation. RTKs are not uniformly activated across the cell membrane but tend to occur upon receptor clustering in specific microdomains (6). DDR1 is not an exception and receptor aggregation has been suggested to participate in its activation (5). Our hypothesis is that in K-Ras-driven lung adenocarcinoma specific membrane microdomains may result in DDR1/Notch clustering facilitating their reciprocal activation. Furthermore, Ras signal output is qualitatively and quantitatively dictated by its own membrane sub-localization (7). Whether K-RasG12V might also be present in such microdomains remains to be determined, but in any case DDR1/Notch local enrichment may favour some post-translational modifications required for their full activity. As an illustrative example, Src is required both for full DDR1 activity as well as for the proteolytic activation of Notch in certain cellular contexts.
This putative DDR1/Notch co-regulation may be particularly relevant in specific tumour sub-populations. Notch and DDR1 have been reported to play a pivotal role to maintain cancer stem cell (CSC) traits in K-Ras driven lung adenocarcinoma and breast cancer respectively (8-10). In this context both DDR1 and Notch are subject to common regulatory mechanisms such as those involving PKC function (9,10) that, incidentally, also controls K-Ras membrane localization (7), again reinforcing the hypothesis that local clustering might facilitate co-regulation. Interestingly, the pharmacological co-inhibition of DDR1/Notch in lung adenocarcinoma PDX driven by K-Ras mutations eliminates the most aggressive tumour component and considerably delays disease relapse (1). This observation could be compatible with the combined DDR1/Notch inhibition being particularly effective on CSCs.
The therapeutic efficacy of DDR1/Notch co-inhibition may not only be a consequence of its effect in the tumour proper but in additional components. In agreement with an important Notch function in endothelial cells, co-inhibition of DDR1/Notch in lung adenocarcinoma resulted in diminished tumour vasculature as measured by CD31 staining (Figure 1A). Whether this is exclusively reliant on Notch inhibition or whether DDR1 also plays an important role in tumour endothelial cells remains to be determined. In any case it is likely that the decreased vascularization contributes to the extensive tumour necrosis observed upon DDR1/Notch inhibition (1).
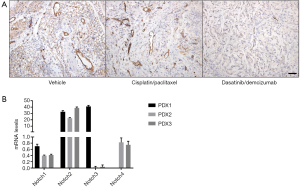
Finally, in his recent commentary Dr. Lendahl speculated on whether the Notch response is quantitatively or qualitatively blunted by DDR1 inhibition. It will be important to identify whether any of the four Notch receptors plays a prominent role in K-Ras-driven lung adenocarcinoma. In our study, Notch2 was over-represented in our limited subset of human PDX samples (Figure 1B). Accordingly, in the TCGA dataset Notch2 is the most frequently deregulated (amplified or up-regulated) isoform in human lung adenocarcinoma. In any case, and as discussed above, we have no evidence of a vertical relationship between the DDR1 and Notch pathways. Instead, our experimental evidences rather favour the existence of a bi-directional interaction. Whether this synergy is limited to their activation on the cell surface or, in addition, their respective downstream signals converge to sustain pathways essential for tumour progression is currently unknown. In this regard, the activation of the MAPK pathway, known to be essential for the development of K-RasG12V-driven lung adenocarcinoma, is only efficiently suppressed when both DDR1 and Notch are simultaneously targeted (1). Whether this synergistic effect can be extended to other signalling and/or pro-survival pathways downstream of oncogenic K-Ras remains to be elucidated.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shaohua Cui (Department of Pulmonary Medicine, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.12.59). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ambrogio C, Gómez-López G, Falcone M, et al. Combined inhibition of DDR1 and Notch signaling is a therapeutic strategy for KRAS-driven lung adenocarcinoma. Nat Med 2016;22:270-7. [Crossref] [PubMed]
- Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development 2011;138:3593-612. [Crossref] [PubMed]
- De La O JP, Emerson LL, Goodman JL, et al. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc Natl Acad Sci U S A 2008;105:18907-12. [Crossref] [PubMed]
- Kim HG, Hwang SY, Aaronson SA, et al. DDR1 receptor tyrosine kinase promotes prosurvival pathway through Notch1 activation. J Biol Chem 2011;286:17672-81. [Crossref] [PubMed]
- Valiathan RR, Marco M, Leitinger B, et al. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev 2012;31:295-321. [Crossref] [PubMed]
- Bethani I, Skånland SS, Dikic I, et al. Spatial organization of transmembrane receptor signalling. EMBO J 2010;29:2677-88. [Crossref] [PubMed]
- Arozarena I, Calvo F, Crespo P. Ras, an actor on many stages: posttranslational modifications, localization, and site-specified events. Genes Cancer 2011;2:182-94. [Crossref] [PubMed]
- Zheng Y, de la Cruz CC, Sayles LC, et al. A rare population of CD24(+)ITGB4(+)Notch(hi) cells drives tumor propagation in NSCLC and requires Notch3 for self-renewal. Cancer Cell 2013;24:59-74. [Crossref] [PubMed]
- Ali SA, Justilien V, Jamieson L, et al. Protein Kinase Cι Drives a NOTCH3-dependent Stem-like Phenotype in Mutant KRAS Lung Adenocarcinoma. Cancer Cell 2016;29:367-78. [Crossref] [PubMed]
- Gao H, Chakraborty G, Zhang Z, et al. Multi-organ Site Metastatic Reactivation Mediated by Non-canonical Discoidin Domain Receptor 1 Signaling. Cell 2016;166:47-62. [Crossref] [PubMed]


