
Metabolites-mitochondria-macrophages (MMM): new therapeutic avenues for inflammation and muscle atrophy
Mills et al. (1) have published an important article to demonstrate that upon lipopolysaccharide (LPS) stimulation, macrophages in mice change to a pro-inflammatory state, producing ATP instead by oxidative phosphorylation (OX-PHOS) to glycolysis pathway. They demonstrate that increased mitochondrial oxidation of succinate through succinate dehydrogenase (SDH) brings high mitochondrial membrane potential and increases production of reactive oxygen species (ROS). Blocking ROS production with rotenone, a complex I inhibitor and uncoupling mitochondria, results in an inhibition of this macrophagic pro-inflammatory phenotype. Also, expressing alternative oxidase, which inhibits the inflammatory phenotype, protects mice from LPS lethality.
From these basic experiments, we might extrapolate consequences that apply to human diseases. For instance, it is interesting to note that dimethyl-fumarate, a cell penetrating fumarate ester, has been recently approved by FDA as a front-line drug for the treatment of relapsing-remitting multiple sclerosis (2), since it boosted both LPS induced IL-1beta expression and TNF-alpha production. It is well known that fumarate can stabilize hypoxia inducible factor (HIF-1alpha) and this is the likely mechanism by which it is induced IL-1beta. Furthermore, previous reports demonstrate that metformin, which inhibits complex I, decreases IL-1beta in response to LPS. Mills et al. (1) demonstrate that succinate oxidation and the high mitochondrial membrane generate a redox signal that can alter HIF-1alpha activity.
Complex I can produce large amount of ROS by the regulated process of reverse electron transport when the CoQ pool is highly reduced. This happens especially during ischemia-reperfusion injury. ROS signal is sensitive to uncoupling, achieved through an inhibitor of succinate oxidation, dimethyl-malonate (1). The central role of mitochondria in immune signaling and in innate immune response fits the function of this organelle in cell death and signaling. In fact, the endosymbiotic origin of mitochondria may reflect not only the requirement of eukaryote to evolve their respiratory oxidation but also the idea that mitochondria might have a role as “signaling hubs”, which can influence the phenotype of immune cells and possibly of other cell types (i.e., T and B lymphocytes and other circulating cells) to respond to infections. Finally, the fact that succinate oxidation is an important regulator of signaling in inflammation, opens a great number of new therapeutic opportunities, since it can be also modulated by a series of metabolites such as dimethyl-malonate, itaconate (3), which modulate succinic dehydrogenase. Inhibitors of SDH might prove useful shifting macrophages away from pro-inflammatory gene expression towards anti-inflammatory gene expression within an inflammatory environment. Therefore, the observation obtained with the LPS model (1) might be extrapolated both to human auto-immune and inflammatory disorders.
I will specially cover the key-possibilities that are opened by these experimental observations in the neuromuscular disorders field, where the mechanism of inflammation is part of myositis, necrotizing autoimmune myopathy, muscular dystrophies, autoimmune neuropathy (both chronic and acute) (4). Furthermore, down-regulation of mitochondria might occur in common muscle diseases, such as muscle ischemia due to peripheral vascular diseases, “intensive care unit” weakness (4), or uncommon diseases such as deconditioning of muscle in McArdle disease (5). Another condition that has probably a role for mitochondria down-regulation is atrophy and cachexia of muscle, which corresponds to a fast atrophy of proximal muscle due to unknown factors released by tumours (such as pancreatic or stomach cancer). In this condition, muscle fibers loose OX-PHOS capacity, patients undergo muscle atrophy and therapeutic intervention are urgently needed. Whether this is a metabolic effect that occurs for remote cancer effect or a paracrine myopathy, remains to be studied.
Metabolic reprogramming in macrophages has been recently observed. Pro-inflammatory stimuli, such as LPS, cause macrophagic alteration and a switch away from oxidative phosphorylation towards glycolysis, similar to Warburg effect in tumour cells (6). Succinate is a TCA-cycle intermediate and activates HIF-1alpha (7) which promotes inflammatory gene expression; on the contrary, endogenous itaconate regulates metabolic remodelling, succinate levels by inhibiting SDH and mitochondrial respiration in inflammatory macrophages. A Table shows the characteristics of these two classes of macrophages, denominated M1 and M2 macrophages (Table 1).
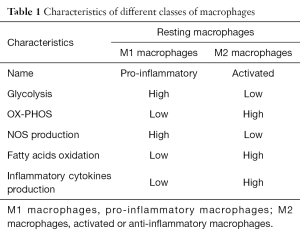
Full table
It has been recognized that macrophages switch as a potential therapeutic target from M1 (pro-inflammatory macrophage) to M2 (anti-inflammatory macrophage) state. This can happen through an action of drugs such as metformin, and rotenone (a complex I inhibitor). While M1 macrophages metabolism features high i-NOS expression and NO production, M2 macrophages have high levels of oxidative phosphorylation and fatty acids oxidation.
Mills et al. (1) have studied the critical enzyme SDH that drives inflammatory response through metabolic repurposing of mitochondria. Can this be exploited therapeutically in chronic inflammatory myopathies and neuropathies (4) or tissue regeneration?
Here we consider that reprogramming macrophages with tricarboxylic acid (TCA) cycle intermediate metabolites or metabolic inhibitors might be a novel therapeutic avenue for the treatment of inflammatory disease (“MMM” strategy).
These classes of metabolites shunt M1 macrophages by changing from a metabolism similar to that described in tumour cells with the Warburg effect (6), i.e., high glycolysis with low aerobic metabolism, to utilization of fatty acids and production of ROS.
The crucial observation is that mitochondrial derangement occurs frequently when immune-mediated cells undergo a drastic metabolic change during an inflammatory process. In fact, inflammation does not only produce a response to infection or inflammation, but also a number of metabolites and cytokines. This response occurs in neuromuscular tissues, for instance, when in muscle increased macrophages are seen in two possible autoimmune inflammatory disorders, i.e., inflammatory myopathies, where macrophages destroy the muscle fibers in acute myositis (Figure 1) and in necrotizing auto-immune myopathy. A similar reactive inflammation might be seen in muscular dystrophies where macrophages might stimulate muscle regeneration after necrosis.
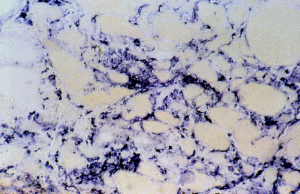
In several animal models, myotoxins are used to study regeneration after the necrosis they induce: therefore one can follow release by macrophages of a series of metabolites and interleukins. In the calpainopathy disease, Amato (8) has demonstrated an eosinophilic inflammation and active macrophages clusters might appear, where increased ROS production is likely to occur.
A similar inflammatory reaction mimicking polymyositis is frequent in dysferlinopathy (9,10), where a secondary mitochondrial dysfunction also occurs (11,12), and the use of steroids appears inappropriate.
Following mitochondrial dysfunction, they are recruited to phagolysosomes and the production of adenine-dinucleotide-phosphate by the pentose phosphate pathway occurs. NADPH is the electron donor for NADPH oxidase, finally transferring electrons to molecular oxygen, thus producing the superoxide ion, a potent ROS species. Conversely, activated macrophages (M2) generate ATP mainly through oxidative phosphorylation. Modulators of these M1-M2 shifts can occur also in inflammatory or pseudo-inflammatory muscle disorders. Industrial companies are highly interested in the production of exogenous itaconic acid, which function as an inhibitor of various enzymes including SDH. As a result of such metabolic intervention, will such compound lead to accumulation of succinic acid, fumaric acid, etc., and act as an immune supportive metabolite? So far, we know that itaconic acid acts as a specific anti-microbial metabolite that supports the cellular metabolic immune response but we have to explore if it has an important role during inflammation and act in addition to TCA-cycle intermediate and their endogenous production (3).
Another anaplerotic substrate is propionyl-carnitine, which is used in secondary carnitine deficiency (13), where it acts as a potential anaplerotic metabolite by transforming in propionyl-CoA. Propionyl-carnitine has been used both in peripheral vascular disease (13) and McArdle disease (5) to increase mitochondrial efficiency, in view of the well known mitochondrial down-regulation that occurs in these two disorders. A muscle deconditioning state occurs in situations where either cardiac pump is less active and blood circulation insufficient, such as in peripheral vascular disease or myocardial infarction, but also in metabolic disorders with a block of glycogenolysis (5) and secondary down-regulation of OX-PHOS capacity.
Can OX-PHOS down-regulation be rescued? We have observed in clinical trials a robust and clinical action of propionyl-carnitine in McArdle disease and in peripheral vascular disease.
Another clinical consequence of the influence of mitochondria on macrophage action and on B-, T-lymphocytes sub-populations is that in mitochondrial disorders it is likely that infections might be more common.
Mitochondrial disorders are a heterogeneous group of diseases, which might be due to defects in OX-PHOS respiratory chain or to a nuclear DNA mutation. Mitochondrial dysfunctions have a role also in Parkinson syndrome, migraine and, possibly, ALS. For their treatment, cocktails composed of vitamins, cofactors and anti-oxidants are used. If a different susceptibility to infections and inflammatory state should be present in these patients, this would require further therapeutic approaches, such as “MMM strategy”.
This susceptibility should be investigated in large mitochondrial patients populations already collected in registries, such as the Italian Mitochondrial Disease Registry, the Australian Mitochondrial Disease Registry, the United Mitochondrial Disease Foundation.
There is also the important question if exposure to hypoxia, especially in older individuals or in obese sedentary subjects with sarcopenia or in cachexia, because of the critically regulatory role of HIF-1alpha, which might show an abnormal response.
On the contrary, high intensity exercise seems to reverse both the possible adverse factor of exposure to hypoxia (14) and in sarcopenia. All these questions awake urgent response to uniform current medical recommendations for such fragile patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hao Feng (Experimental Surgical Research, Department of General, Visceral, Transplant, Vascular and Thoracic Surgery, Hospital of the LMU Munich, Germany).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.01.37). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mills EL, Kelly B, Logan A, et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016;167:457-470.e13. [Crossref] [PubMed]
- Zhovtis Ryerson L, Green R, Confident G, et al. Efficacy and tolerability of dimethyl fumarate in White-, African- and Hispanic- Americans with multiple sclerosis. Ther Adv Neurol Disord 2016;9:454-61. [Crossref] [PubMed]
- Lampropoulou V, Sergushichev A, Bambouskova M, et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab 2016;24:158-66. [Crossref] [PubMed]
- Angelini C. editor. Acquired Neuromuscular Disorders: Pathogenesis, Diagnosis and Treatment. Switzerland: Springer International Publishing, 2016
- Martinuzzi A, Schievano G, Vergani L, et al. Diagnosis and therapy of myophosphorylase deficiency: experience with a group of Italian patients. Basic Appl Myol 1996;6:107-14.
- Warburg O. On the origin of cancer cells. Science 1956;123:309-14. [Crossref] [PubMed]
- Corcoran SE, O'Neill LA. HIF1α and metabolic reprogramming in inflammation. J Clin Invest 2016;126:3699-707. [Crossref] [PubMed]
- Amato AA. Adults with eosinophilic myositis and calpain-3 mutations. Neurology 2008;70:730-1. [Crossref] [PubMed]
- Fanin M, Angelini C. Muscle pathology in dysferlin deficiency. Neuropathol Appl Neurobiol 2002;28:461-70. [Crossref] [PubMed]
- Fanin M, Angelini C. Progress and challenges in diagnosis of dysferlinopathy. Muscle Nerve 2016;54:821-35. [Crossref] [PubMed]
- Vincent AE, Rosa HS, Alston CL, et al. Dysferlin mutations and mitochondrial dysfunction. Neuromuscul Disord 2016;26:782-8. [Crossref] [PubMed]
- Liu F, Lou J, Zhao D, et al. Dysferlinopathy: mitochondrial abnormalities in human skeletal muscle. Int J Neurosci 2015; [Epub ahead of print]. [Crossref] [PubMed]
- Brevetti G, Fanin M, De Amicis V, et al. Changes in skeletal muscle histology and metabolism in patients undergoing exercise deconditioning: effect of propionyl-L-carnitine. Muscle Nerve 1997;20:1115-20. [Crossref] [PubMed]
- Tsai HH, Chang SC, Chou CH, et al. Exercise Training Alleviates Hypoxia-induced Mitochondrial Dysfunction in the Lymphocytes of Sedentary Males. Sci Rep 2016;6:35170. [Crossref] [PubMed]


