
Osimertinib: a breakthrough for the treatment of epidermal growth factor receptor mutant lung adenocarcinoma
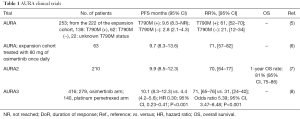
At the time of acquired resistance to the first generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), gefitinib, erlotinib or afatinib (1), 50–60% of EGFR mutant non-small cell lung cancer (NSCLC) patients have developed the secondary gatekeeper T790M point mutation on exon 20 of the EGFR gene (2,3). Osimertinib (AZD9291) is a third-generation EGFR TKI that targets EGFR mutant (including T790M positive) tumors (4). It was initially evaluated in EGFR mutant NSCLC patients who had disease progression after previous treatment with an EGFR TKI, in the phase I part of the phase I/II AURA study (5). A response rate of 51% was achieved for all patients treated across all dose levels. Among the 222 patients of the expansion cohorts, the response rate to osimertinib was 61% for EGFR T790M-positive patients while the EGFR T790M-negative patients were not able to achieve a response rate greater than 21% (Table 1). No dose limiting toxicities were observed at any dose level and, based on tumor growth inhibition, the dose of 80 mg once daily was selected for being further evaluated for the treatment of EGFR T790M-positive NSCLC patients (5). On November 13th 2015, and on February 3rd 2016, FDA and EMA approved osimertinib 80 mg once daily for the treatment of EGFR T790M-positive NSCLC patients, respectively, based on the data from two phase II studies (AURA extension and AURA2) and the AURA phase I expansion study. Sixty-three EGFR T790M-positive, dose expansion cohort patients receiving 80 mg of osimertinib once daily in the phase I part of the AURA study demonstrated an objective response rate (ORR) of 71% [95% confidence interval (CI), 57–82] and a median progression-free survival (PFS) of 9.7 months (95% CI, 8.3–13.6). In a pre-planned pooled analysis of the AURA extension phase II study and the AURA2 phase II study with a total of 411 EGFR T790M-positive patients, ORR was 66% and median PFS was 11.0 months (95% CI, 9.6–12.4) (6).
Full table
Goss et al., published in Lancet Oncology the final results of the phase II, open-label, single-arm AURA2 study, which assessed the efficacy and safety of osimertinib in patients with EGFR T790M-positive NSCLC, who had progressed after previous therapy with EGFR TKIs. In less than 6 months, more than 472 patients were screened, 210 EGFR T790M-positive patients were treated with osimertinib and 199 patients were evaluable for response analysis in the AURA II study (7). The FDA approved Cobas EGFR mutation test v2 was used for the central confirmation of the EGFR T790M mutation. An ORR of 70% and a disease control rate of 92% were achieved, as evaluated by blinded independent central review. Six (3%) patients achieved complete response with osimertinib treatment and 134 (67%) achieved partial response. The median duration of response was 11.4 months (95% CI, 9.0–not calculable). There was a high concordance between the responses obtained by investigator assessment and by the blinded independent central review. The median PFS was 9.9 months (95% CI, 8.5–12.3) (7). The treatment was well tolerated with the most common treatment related grade 3–4 adverse events being prolonged electrocardiogram QT, decreased neutrophil count, and thrombocytopenia. Interstitial lung disease occurred in 2% of the patients.
Only 2 months after the publication of the AURA2 study, the results of the phase III AURA3 clinical trial became available (8). In this study, 419 EGFR T790M-positive NSCLC patients who had progressed during first-line were assigned in a 2:1 ratio to receive osimertinib or platinum-pemetrexed chemotherapy. Median PFS was significantly longer with osimertinib compared with chemotherapy [10.1 vs. 4.4 months; hazard ratio (HR) 0.30; 95% CI, 0.23–0.41; P<0.001] (8). Osimertinib-treated patients achieved an ORR of 71% (95% CI, 65–76) in comparison to an ORR of 31% (95% CI, 24–40) for those who received chemotherapy (odds ratio for objective response, 5.39; 95% CI, 3.47–8.48; P<0.001) (8).
It was previously demonstrated that patients with the T790M mutation detected by plasma ctDNA respond equally to osimertinib as those whose mutation is detected in a tumor tissue biopsy (9-11). Similar findings were reported in the AURA3 trial (8). On September 29th, 2016, the FDA approved an expansion of the Cobas EGFR blood mutation test v2 to include testing of the T790M mutation in order to confirm the presence of the EGFR T790M mutation and qualify patients for treatment with osimertinib. Due to the relatively high false negative rates with plasma T790M testing, it is highly recommended that patients with a negative liquid biopsy for the presence of the T790M to be reevaluated for the feasibility of a tissue biopsy (10).
More than 30% of EGFR mutant NSCLC patients, whose disease progressed during or after first line EGFR TKI, have brain metastases (12). In comparison with other EGFR TKIs, including gefitinib, rociletinib or afatinib, osimertinib has demonstrated greater blood-brain barrier penetration in preclinical models (13). In the AURA2 trial, patients with brain metastases obtained an ORR of 69% (95% CI, 58–79) and a median PFS of 9.2 months (95% CI, 7.7–11.1) (7). In the AURA3 study, among the 144 patients who had central nervous system (CNS) metastases, median PFS was 8.5 months (95% CI, 6.8–12.3) for those treated with osimertinib and 4.2 months (95% CI, 4.1–5.4) for those who received chemotherapy (HR 0.32; 95% CI, 0.21–0.49) (8). Interestingly, due to the high risk of development CNS metastases in EGFR mutant NSCLC, an agent with high blood-brain barrier penetration, AZD3759, has been recently developed and is currently being evaluated in a phase I clinical trial (14). Unavoidably, as happens with the first generation EGFR TKIs, osimertinib treated patients develop resistance to treatment after less than one year (8). An additional EGFR mutation, the C797S, can cause resistance to third generation EGFR TKIs and its allelic context can define sensitivity to subsequent treatments (15,16) (Figure 1).
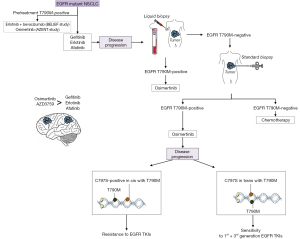
Osimertinib is now being evaluated in the first line setting of EGFR mutant NSCLC patients. According to the results of two (80 and 160 mg) phase I expansion cohorts of the AURA study including 6 EGFR mutant patients, an overall median PFS of 19.3 months (95% CI, 13.7–not reached) and an ORR of 77% (95% CI, 64–87) were obtained (17). The phase III FLAURA study (NCT02296125) compares osimertinib with gefitinib or erlotinib in treatment-naïve EGFR mutant NSCLC patients. PFS in patients with tumors harboring T790M is a key secondary objective of the study (18). The coexistence of the pretreatment T790M mutation has been under appreciated, in spite of accumulative evidence that it is present in a frequency of 35–60%, using different detection methods (19-21). In the Spanish Lung Cancer Group (SLCG) and the European Thoracic Oncology Platform (ETOP) BELIEF trial, pretreatment T790M mutations were centrally identified in 34% of the patients who reached a median PFS of 16 months (95% CI, 13.1–not reached) with the combination of erlotinib plus bevacizumab (21). We have recently started the AZENT study (NCT02841579), an investigator initiated study that explores the safety and efficacy of osimertinib as first line therapy for patients with metastatic EGFR mutant NSCLC and concomitant pretreatment T790M mutation (Figure 1).
Without any doubt, osimertinib has made a breakthrough in lung cancer therapy. Other third generation EGFR TKIs are in clinical development, including olmutinib (Hanmi Pharmaceutical Company), EGF816 (Novartis Pharmaceuticals), naquotinib (Astellas Pharma Global Development), PF06747775 (Pfizer) and avitinib (Hangzhou ACEA Pharmaceutical Research) (22,23). Still, there are several issues to be resolved in the treatment of EGFR mutant patients. One important issue is the discovery of the best therapeutic approach, besides chemotherapy, for those patients who are not EGFR T790M-positive at the time of progression to first and second generation EGFR TKIs. A second issue is that, even if osimertinib is an efficient treatment for T790M driven acquired resistance to initial EGFR inhibition, still the number of complete responses reported in the phase II and III clinical trials is very small, indicating that we are far from the cure of this disease. We have shown that co-targeting STAT3 and Src with EGFR can more efficiently abrogate tumor growth than EGFR inhibition alone (24). Resistance to first, second and third EGFR TKIs are heterogeneous and complex, evolving dynamically under the pressure of each generation’s inhibitor and is a challenge for the development of novel targeted combinations.
Acknowledgments
Funding: This work was funded by La Caixa Foundation and Red Tematica de Investigacion Cooperativa en Cancer (RTICC; grant RD12/0036/ 0072).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shaohua Cui (Department of Pulmonary Medicine, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.01.18). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee JK, Hahn S, Kim DW, et al. Epidermal growth factor receptor tyrosine kinase inhibitors vs conventional chemotherapy in non-small cell lung cancer harboring wild-type epidermal growth factor receptor: a meta-analysis. JAMA 2014;311:1430-7. [Crossref] [PubMed]
- Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73 [Crossref] [PubMed]
- Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786-92. [Crossref] [PubMed]
- Greig SL. Osimertinib: First Global Approval. Drugs 2016;76:263-73. [Crossref] [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Yang J, Ramalingam SS, Jänne PA, et al. LBA2_PR: Osimertinib (AZD9291) in pre-treated pts with T790M-positive advanced NSCLC: updated Phase 1 (P1) and pooled Phase 2 (P2) results. J Thorac Oncol 2016;11:S152-3. [Crossref] [PubMed]
- Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016;17:1643-52. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Oxnard GR, Thress KS, Alden RS, et al. Association Between Plasma Genotyping and Outcomes of Treatment With Osimertinib (AZD9291) in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:3375-82. [Crossref] [PubMed]
- Rosell R, Karachaliou N. Implications of Blood-Based T790M Genotyping and Beyond in Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:3361-2. [Crossref] [PubMed]
- Sacher AG, Paweletz C, Dahlberg SE, et al. Prospective Validation of Rapid Plasma Genotyping for the Detection of EGFR and KRAS Mutations in Advanced Lung Cancer. JAMA Oncol 2016;2:1014-22. [Crossref] [PubMed]
- Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res 2010;16:5873-82. [Crossref] [PubMed]
- Ballard P, Yates JW, Yang Z, et al. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin Cancer Res 2016;22:5130-40. [Crossref] [PubMed]
- Yang Z, Guo Q, Wang Y, et al. AZD3759, a BBB-penetrating EGFR inhibitor for the treatment of EGFR mutant NSCLC with CNS metastases. Sci Transl Med 2016;8:368ra172 [Crossref] [PubMed]
- Niederst MJ, Hu H, Mulvey HE, et al. The Allelic Context of the C797S Mutation Acquired upon Treatment with Third-Generation EGFR Inhibitors Impacts Sensitivity to Subsequent Treatment Strategies. Clin Cancer Res 2015;21:3924-33. [Crossref] [PubMed]
- Rosell R, Karachaliou N. Lung cancer: Using ctDNA to track EGFR and KRAS mutations in advanced-stage disease. Nat Rev Clin Oncol 2016;13:401-2. [Crossref] [PubMed]
- Ramalingam S, Yang JC, Lee CK, et al. LBA1_PR: Osimertinib as first-line treatment for EGFR mutation-positive advanced NSCLC: updated efficacy and safety results from two Phase I expansion cohorts. J Thorac Oncol 2016;11:S152. [Crossref] [PubMed]
- Ramalingam SS, Rukazenkov Y, Thomas K, et al. A randomized, phase III study (FLAURA) of AZD9291, a novel EGFR-TKI, versus gefitinib or erlotinib in treatment-naïve patients with advanced non-small cell lung cancer and an EGFR-TKI-sensitizing mutation. J Clin Oncol 2015;33: (suppl; abstr TPS8102).
- Rosell R, Molina MA, Costa C, et al. Pretreatment EGFR T790M mutation and BRCA1 mRNA expression in erlotinib-treated advanced non-small-cell lung cancer patients with EGFR mutations. Clin Cancer Res 2011;17:1160-8. [Crossref] [PubMed]
- Costa C, Molina MA, Drozdowskyj A, et al. The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial. Clin Cancer Res 2014;20:2001-10. [Crossref] [PubMed]
- Stahel RA, Dafni U, Gautschi O, et al. A phase II trial of erlotinib (E) and bevacizumab (B) in patients with advanced non-small-cell lung cancer (NSCLC) with activating epidermal growth factor receptor (EGFR) mutations with and without T790M mutation. The Spanish Lung Cancer Group (SLCG) and the European Thoracic Oncology Platform (ETOP) BELIEF trial. Eur J Cancer 2015;51:S711-2. [Crossref]
- Park K, Lee JS, Han JY, et al. 1300: Efficacy and safety of BI 1482694 (HM61713), an EGFR mutant-specific inhibitor, in T790M-positive NSCLC at the recommended phase II dose. J Thorac Oncol 2016;11:S113. [Crossref] [PubMed]
- Tan CS, Cho BC, Soo RA. Next-generation epidermal growth factor receptor tyrosine kinase inhibitors in epidermal growth factor receptor -mutant non-small cell lung cancer. Lung Cancer 2016;93:59-68. [Crossref] [PubMed]
- Rosell R, Chaib I, Karachaliou N, et al. YAP-NOTCH and STAT3 Signaling Rebound as a Compensatory Response to Gefitinib or Osimertinib Treatment in EGFR Mutant Lung Cancer. J Thorac Oncol 2017;12:S281-2. [Crossref]


