
Advances on immunotherapy in genitourinary and renal cell carcinoma
Introduction
Genitourinary (GU) cancers are a heterogeneous population of neoplasms, the majority of which are prostate, urothelial, and renal cell carcinoma (RCC). These, along with rarer malignancies of the urine excretion tract, have historically represented some of the most challenging malignancies to manage.
Prostate cancer is the second most common cause of cancer in men worldwide, with an estimated 181,000 new cases, and 26,100 deaths in the United States in 2016 (1,2). Localized cases of these malignancies are ultimately treated with a multi-disciplinary modality: surgery, radiation, and/or androgen deprivation with curative intent. Meanwhile, de novo or recurrent advanced stage disease had carried a high mortality rate due to poor durable remissions and second-line therapy options. Current treatment of metastatic prostate cancer focuses on the use of androgen deprivation therapy (ADT) in combination with chemotherapy (docetaxel). Men who progress to castration-resistant prostate cancer (CRPC) recently have several new agents for continued treatment. The FDA has approved molecules that target the androgen-signaling pathway, attenuating the hormone stimulus for prostate cancer survival.
Of kidney neoplasms, RCC accounts for the overwhelming majority with approximately 62,000 new diagnoses yearly (2). About half of all new diagnoses are stage I at discovery while 20% are metastatic. Of those that are metastatic, the 5-year survival rate is 12% (3). Similar to bladder cancer, smoking is a risk factor for RCC as is obesity, hypertension, Von Hippel-Lindau and cystic kidney diseases (4). Localized disease is cured with surgical nephrectomy with or without lymphadenectomy. Cryoablation, radiofrequency ablation, or partial nephrectomy are therapeutic options for smaller masses. Metastatic disease is often treated with nephrectomy as previous studies analyzing interferon alpha-2b (INF) therapy showed an overall survival (OS) increase of approximately 6 months with resection + IFN versus IFN alone. In addition, there have been reports of cytoreductive nephrectomy stimulating an immune response that also targets metastatic disease burden. Many molecular targeted therapies have made it to market within the last decade targeting the tyrosine kinase pathways associated with VEGF, PDGF, mTOR, and AKT. It is only recently have immunotherapeutic agents been integrated into treatment regiments.
Each year bladder cancer affects 74,000 patients with a male to female predominance of 3:1 (5). Despite the large incidence, the mortality is estimated at 16,000 a year due to detection of early, local neoplasms rather than metastatic disease. Cigarette smoking, work related toxin exposure, recurrent urinary tract infections, cyclophosphamide and Lynch Syndrome increase the risk of bladder cancer. Non-muscle invasive bladder cancers were amongst the first cancers to be treated with immunotherapy (6). More advanced muscle invasive bladder cancers have a multidisciplinary approach including surgical resection and neo/adjuvant chemotherapy.
Over the last decade, an improvement in the understanding of tumor biology and how these and other cancers interact with the immune system has markedly changed the way many solid malignancies have been managed. For decades there had been no second line treatment options for metastatic bladder cancer patients who were ineligible or progressed through platinum-based chemotherapy. As with many other types of cancers, the release of a restricted immune system via immune checkpoint inhibition is currently revolutionizing the field of genitourinary cancers. These monoclonal antibodies are developed to remove the ‘brakes’ associated with immune system downregulation. Thus with increased surveillance of the tumor, cytotoxic cells can engage and destroy malignant tumorigenic cells. Immune checkpoints consist of various inhibitory signaling pathways that regulate the duration and amplitude of immunologic responses to tumor cells to avoid excessive damage to peripheral tissue. In 2011, ipilimumab, a CTLA-4 inhibitor, was approved by the FDA for the treatment of metastatic melanoma, making it the first immune checkpoint inhibitor used to treat solid malignancies (7). More recently, programmed death-1 (PD-1) and its ligand (PD-L1) were found to be intricate members of the cellular immunity against neoplastic cells (8). In an activated, unbound state, these molecules permit T-cell activation and cytotoxic killing of cancer cells. However, when they are linked, cellular immunity is attenuated and local cancer cells are permitted the opportunity to proliferate and invade (9). A novel class of monoclonal antibodies called checkpoint inhibitors have been developed which stop PD-1 linkage and thus uncouple the ‘stop’ signal of these neoplastic regulatory cells.
Other methods of immune surveillance, the development of chimeric T-cell receptors and cancer vaccines, have helped shape this distinct class of cancer treatments known as ‘immunotherapies’, which have begun to alter the approach to the way various malignancies are treated. The use of immunotherapy is currently being introduced into the field of genitourinary cancer, with many agents making a noticeable impact on overall and progression free survival. Additionally, the overall well being of patients that have been diagnosed with advanced stage disease has improved with non-chemotherapeutic approaches. Although toxicities such as nausea and vomiting are reduced with this class of drugs, there are immunotherapy-specific adverse effects that must be understood and evaluated by oncologists and patients during treatment. This article will review current treatment strategies for GU cancer and the way that immunotherapy is changing their management.
Prostate cancer immunotherapy
Vaccines
Sipuleucel-T
In 2010, Sipuleucel-T (Provenge®, Dendreon) became the first therapeutic cancer vaccine to be approved by the FDA after it was shown to prolong survival in patients with castration resistant prostate cancer (10). This vaccine consists of autologous dendritic cells that stimulate a T-cell response against prostatic acid phosphatase (PAP), an antigen that is found in 95% of prostate cancers. The vaccine is prepared by harvesting the patient’s own dendritic cells (and other mononuclear cells) via leukapheresis from the peripheral blood. These cells are then exposed ex-vivo to a recombinant fusion protein called PA2024, which consists of PAP fused to granulocyte/macrophage-colony stimulating factor (GM-CSF). An incubation period of 48 hours allows the dendritic cells sufficient time to process the protein for antigen presentation, while GM-CSF leads to the expression of various pro-inflammatory signaling molecules and receptors. These primed dendritic cells are ultimately reintroduced into the patient to stimulate a T-cell response against the cancer cells. These infusions are usually performed every 2 weeks, for a total of three times.
The efficacy of the vaccine was demonstrated in three phase III trials. D9901, published in 2006, was a randomized, double-blind, placebo-controlled trial with progression-free survival (PFS) as its primary endpoint. Although the PFS improvement was insignificant (11.7 months in treated group vs. 10.0 months in placebo group, hazard ratio (HR) =1.45, 95% CI: 0.99–2.11; P=0.052), the secondary endpoint, overall survival, was longer in the treated group than the placebo group (25.9 vs. 24.1 months) (11). D9902A was not completed, as the study was similar to D9901, which was published first showing no statistical improvement in the primary endpoint. A combined analysis was performed from these two studies with the 225 men enrolled that again demonstrated no statistically significant increase in progression-free survival, but did confirm an increase in overall survival [23.2 months in treated group, vs. 18.9 months in placebo group, HR =0.67, 95% CI: 0.49–0.91] (12). A third trial, Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT), was run to corroborate these findings, in which 512 patients were enrolled in a double-blind, placebo-controlled study in which overall survival was the primary, rather than secondary endpoint, confirming a statistically significant increase of 4.1 months (25.8 in the treated group vs. 21.7 in the placebo group, P=0.03) (13). The patients enrolled in each of the three trials above had been diagnosed with metastatic, castration-resistant prostate cancer (mCRPC) with ECOG status 0 or 1, without evidence of visceral metastases, pathologic fractures, or opioid analgesia requirement.
Currently, the clinical role of Sipuleucel-T is restricted to patients with asymptomatic, or minimally symptomatic mCRPC (ECOG status 0 or 1). Because progression of disease and PSA level are unaffected by administration of this vaccine, there are no objective criteria to determine whether a patient is benefiting from treatment or may require an alternative therapy, which is a common reason cited to use other systemic therapies in this patient population.
Prostvac
Prostvac (Bavarian Nordic) is a heterologous prostate cancer vaccine series that consists of a prime-boost regimen involving recombinant vaccinia and fowlpox viruses (14). These vectors have been engineered to encode for transgenes expressing human PSA, and the costimulatory molecules, CD54, CD58, and CD80, which are collectively referred to as TRICOM. Treatment is initiated by subcutaneous administration of PSA-TRICOM via the vaccinia vector, followed by 6 subsequent boosting doses of PSA-TRICOM via the fowlpox vector. Small doses of G-CSF are co-administered with each dose of the vaccine. Completion of this vaccine series leads to enhanced T-cell activation and recognition of PSA-expressing prostate cancer cells. A phase II randomized, controlled, and blinded clinical trial involving 125 patients with minimally symptomatic mCRPC demonstrated an increased overall survival in the treated group compared with the placebo group (25.1 vs. 16.6 months, P=0.006) (15). A phase III randomized, double-blind efficacy study, the PROSPECT trial (NCT01322490), is currently underway to validate the results of the phase II trial.
GVAX
GVAX (Biosante Inc.) is a vaccine consisting of whole, allogeneic, tumor cells. Two prostate cancer cell lines, PC-3 and LNCaP, are transduced with a retrovirus containing cDNA for GM-CSF. The vaccine is administered as one initial dose, followed by boosters every two weeks for 6 months. The results of a phase I/II clinical trial published in 2007 demonstrated an increase in overall survival, which was highest for the group of patients treated with the highest booster dose (34.9 months), followed by the lower booster dose (26.2 months), and then the group that was treated with radiotherapy alone (24 months) (16). However, two phase III clinical trials (VITAL-1 and VITAL-2) had to be discontinued prematurely when it was found that patients in the treated group had an increased rate of mortality, and did not benefit from significant therapeutic effects (17,18). Currently there is no FDA approved indication for the use of GVAX, although further clinical studies involving combination immunotherapy with this vaccine are currently underway for other types of solid malignancies (NCT02243371, NCT02451982).
Immune checkpoint inhibitors
Nivolumab: PD-1 monoclonal antibody
Nivolumab (Opdivo®, Bristol-Myers-Squibb) is a human IgG4 monoclonal antibody that binds to PD-1 expressed on the surface of T-cells. Many solid tumor cells express PD-L1 and PD-L2, which would otherwise bind to PD-1 on T-cells, activating apoptotic pathways leading to T-cell directed death. The antibody prevents this interaction, allowing for a greater T-cell response against cancer cells, and thus making it a checkpoint inhibitor. A trial involving patients with various GU malignancies was initiated to evaluate the response of these tumors to nivolumab, but only 17 patients with CRPC were enrolled, and only one of these patients demonstrated improvement with a reduction in tumor size (19). The focus of this agent has mainly been on the concept of combination therapy with other immunotherapies such as ipilimumab. A particular area of interest has been invested in the use of these agents for treatment of patients with androgen receptor (AR) splicing variants that do not respond to other pharmacologic agents, such as abiraterone and enzalutamide. There is currently a phase II study (NCT02601014) involving 15 men with mCRPC with the AR-V7 splice variant detected on PCR analysis on study, with the primary endpoint being their PSA50 response rate. Although there is currently no FDA approval for the use of any checkpoint inhibitor in prostate cancer, these studies will hopefully uncover potential benefits.
Ipilimumab: CTLA-4 monoclonal antibody
Ipilimumab (Yervoy®, Bristol-Myers-Squibb) is a human monoclonal antibody that binds to CTLA-4 on the surface of T-cells. Under normal circumstances, CTLA-4 competes with costimulatory molecules (B7.1 and B7.2) for binding to CD28 on the T-cell surface, which plays a role in activating signaling pathways crucial to T-cell activation along with the T-cell receptor. Ipilimumab prevents this interaction, allowing for greater activation of CD28, and ultimately T-cell functioning (20). A phase III trial looked at 799 men with mCRPC and at least one bone metastasis who progressed after being treated with docetaxel: 399 of which were treated with ipilimumab, and 400 with placebo. Although the treated group did show an increase in median overall survival (11.2 months) in comparison to the placebo group (10.0 months), this difference was not statistically significant (HR =0.85, 0.72–1.00; P=0.053) (21). The most common adverse events were diarrhea, colitis, anemia, and fatigue (grade 3–4) (22) (Figure 1).
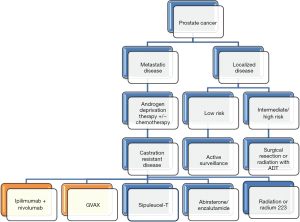
Urothelial carcinoma immunotherapy
Induced inflammatory response
The majority of bladder cancers are within the lumen of the bladder and non-metastatic at diagnosis. For non-muscle invasive bladder cancer, transurethral resection is the modality of choice followed by intravesicular therapy. Bacille Calmette-Guerin (BCG), a live attenuated strain of Mycobacterium Bovis, in 6 weekly bladder installations followed by maintenance therapy decreases recurrence and limits progression (23). BCG induces an inflammatory response within the bladder lumen that permits cytotoxic T-Cell clearance of carcinoma in situ and was FDA approved in the 1980s. Should cystoscopy show continued disease after induction and maintenance, another 6-week course can commence. Many international organizations site evidence that suggests maintenance every 3 months for a minimum of 1 year may reduce recurrence as well as progression (24,25). Adverse effects are due to the cumulative irritative effects on the bladder lumen. Other side effects include flu-like symptoms: fever, arthralgias, gross hematuria, and elevated LFTs. Rare severe effects can cause respiratory distress, mental status changes, and distributive shock (26).
Immune checkpoint inhibitors
Atezolizumab: PD-L1 monoclonal antibody
Bladder cancer has the 4th highest mutational gene burden behind melanoma, lung squamous carcinoma, and lung adenocarcinoma (27). Due to this and the success of immunotherapy in skin and lung cancer, immune checkpoint inhibitors were evaluated for the treatment of bladder cancer. Atezolizumab (Tecentriq®, Genentech), a humanized immunoglobulin monoclonal antibody against PD-L1 was approved by the FDA in early 2016. This approval was based upon the Phase II ‘IMvigor 210’ trial in patients with inoperable, locally advanced or metastatic disease that progressed during or within 12 months of first line platinum-containing chemotherapy (second line) (28). It enrolled 310 patients and their tumors were assessed for PD-L1 expression on infiltrating immune cells in the tumor microenvironment [either less than 1% (IC0), 1% to 5% (IC1) or over 5%(IC2/3)]. Each patient received 1200mg of atezolizumab IV every 3 weeks until toxicity or radiologic/clinical progression. The primary endpoint of objective response rate (ORR) was seen in 26% of the IC2/3 group, 18% in the IC1/2/3 groups, and 15% overall over a one year period. Of those 45 patients with an initial response, 84% had ongoing responses at 11.7 months. The most common side effects (rate >20%) included fatigue, low appetite, nausea, urinary tract infections, and constipation. Rare side effects include organ inflammation of lung, liver, colon, pancreas, and endocrine organs. In addition, severe infections and immune-related dysfunction (Guillain-Barre, encephalitis, myasthenia gravis) were also reported in addition to teratogenic effects.
Avelumab: PD-L1 monoclonal antibody
Phase 1 clinical trials are ongoing with avelumab (Pfizer/Merck), a PD-L1 inhibitor, as a second line therapy in metastatic urothelial cancer. Results of the JAVELIN trial reported at ASCO 2016 found patients who received avelumab at 10mg/kg IV every 2 weeks had ORR of 18.2% (8 of 44 patients), with 2 complete remissions and 6 partial remissions (29). Similar to atezolizumab, those patients with greater PD-L1 expression (greater than 5%) had an increased ORR of 50% versus 4.3% and PFS rate at 6 months of 58.3% versus 16.6%. OS at 12 months was 50.9% for all patients. A Phase III trial has been initiated for avelumab in the first-line setting for patients with locally advanced or metastatic urothelial cancer (NCT01772004).
Nivolumab: PD-1 monoclonal antibody
The PD-1 inhibitor nivolumab (Opdivo®, Bristol-Myers-Squibb) was the second immune checkpoint inhibitor to be granted FDA breakthrough therapy for advanced urothelial carcinoma. In June of 2016, it was approved for unresectable locally advanced or metastatic urothelial carcinoma after progression on a platinum-based regimen (second line). This designation was based upon two studies the CHECKMATE-275 and -032 studies. The -275 Phase II study of 265 patients showed an ORR of 19.6%, a median PFS of 2 months, and a median OS of 8.74 months (22). Even with those patients whose PD-L1 expression was <1%, the objective response rate (16.1% versus 23.8% over 1% expression) was higher than chemotherapy. The response duration was not reached over a period of 7 months. Importantly, this study did not evaluate sub-group OS of those patients with less than 1% PD-L1 expression.
The CHECKMATE-032 trial of 78 patients was presented at ASCO in June 2016 confirming nivolumab monotherapy in metastatic urothelial cancer refractory to platinum-based chemotherapy (30). These patients were found to have an ORR of 24.4%, an OS of 9.72 months, and PFS of 2.78 months, surpassing the original study. Clinically, patients received 3 mg/kg IV nivolumab every 2 weeks until progression. If a patient progressed, they had the option of continuing with nivolumab combined with ipilimumab (Yervoy). Importantly this trial observed that a majority of patients (62.7%) had PD-L1 expression levels less than 1% as evaluated by the Dako PD-L1 IHC 28-8 pharmDx kit. Towards that point, expression did not effect the clinical activity of nivolumab (PD-L1 >1% ORR =24%; PD-L1 <1% ORR =26.2%). Similar adverse effects to other studies included fatigue, pruritus, and elevated LFTs. The data regarding addition of ipilimumab after progression has yet to be released.
Pembrolizumab: PD-1 monoclonal antibody
The randomized Phase III KEYNOTE-045 study of the PD-1 inhibitor pembrolizumab (Keytruda®, Merck, at 200 mg every 3 weeks) in previously treated advanced urothelial cancer met the primary endpoint of OS when compared to physician-choice chemotherapy regimens (31). The trial was stopped early due the overall efficacy of the drug as well as there being no additional, unknown side effects (versus already known PD-1 adverse effects). The population studied included those patients who were metastatic, locally advanced, or unresectable who had previously progressed on a platinum-based chemotherapy (second line). The regimens pembrolizumab was superior to included paclitaxel (175 mg/m2 every 3 weeks), docetaxel (75 mg/m2 every 3 weeks), and vinflunine (320 mg/m2 every 3 weeks).
The KEYNOTE-052 study is currently evaluating the efficacy of pembrolizumab as an active first-line therapy for locally advanced or metastatic bladder cancer patients who are unable to use frontline cisplatin-based chemotherapy (32). Initial results of the first 100 patients were stated at the ESMO 2016 meeting. The primary endpoint, objective response rate, was found to be 24%. This study analyzed the tumor PD-L1 expression on either immune cells or tumor cells and found likely responders (37%) to harbor at least 10% PD-L1. Although the OS rate was lower than chemotherapeutic controls, the duration of response was longer in cisplatin-ineligible patients.
Ongoing trials include KEYNOTE-057 (NCT02625961) evaluating pembrolizumab in non-invasive urothelial cancer unresponsive to BCG as well as KEYNOTE-361 (NCT02853305) evaluating pembrolizumab in combination with platinum chemotherapy in advanced or metastatic urothelial cancer (Figure 2).
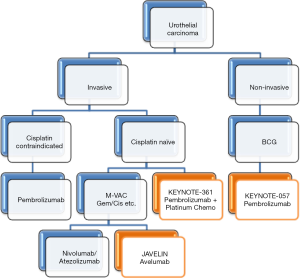
RCC immunotherapy
CYTOKINES
IL-2
Despite the trend towards molecular inhibitors in the last decade, immunotherapy was the primary treatment for patients with advanced or metastatic RCC. Induction of a chemokine ‘storm’ with high dose IL-2 was first trialed in an attempt to form such a strong immunologic reaction, that tumor cells would be eradicated after the end of therapy. In Phase II and Phase III clinical trials, high dose human recombinant IL-2, aldesleukin (Proleukin®, Prometheus) was found to induce long-term remissions in carefully selected patients (33,34). Further, the SELECT trial found that IL-2 could induce objective response rates (ORR) in metastatic RCC of 30% if they were of clear cell histology (35). Durable responses in up to 5% (especially in those with good performance status or younger age) were seen for up to three years. Trials comparing high-intensity inpatient treatment versus low dose outpatient treatment found improved response rates and complete remissions in the high dose group although no significant difference in overall survival was found (36). Implementing a treatment regimen of 600,000 international IL-2 units per kilogram, every 8 hours for 5 days and for two total cycles, complete responses were obtainable (23 of 259 patients, 9%) (37). Importantly, prior nephrectomy within 6 months of immunotherapy increased the three-year survival rate to 46% (38). These responses come at a large cost with increased vascular permeability causing multi-organ dysfunction, severe hypotension and even death (39). Although inpatient ICU settings are capable of supporting the patient through these adverse effects, patient selection is of the utmost importance before initiating treatment. Recently groups are in early studies investigating human IL-10 in RCC both alone and in combination with anti-PD-1 pembrolizumab (see below) (40).
INTERFERON-α
Many trials analyzed the ability of IFN-α as a single agent in metastatic RCC as well as in combination with IL-2, VEGF-inhibitors, and chemotherapy. Only IFN-α alone was shown to improve OS by 3.8 months and had a similar long-term remission to IL-2 with significantly less toxicity (41). The highest OR seen was 15% within a four month period. Most trials saw an OR of 7.5% and rarely did a durable remission occur greater than 1 year. A study of 174 patients treated with INF-α demonstrated a one year survival of 12% with median OS of 8.5 months (42). These studies also carefully selected patients who were capable of proceeding with the intense treatment regimen, observing poor responses in those with any metastases, liver involvement or an interval metastatic progression of less than 1 year (41). Overall, IFN-α therapy has been replaced with the advent of molecular targeting and immune checkpoint inhibitors.
Immune checkpoint inhibitors
Nivolumab: PD-1 monoclonal antibody
Early immunotherapy studies in RCC were based on the understanding that most patients progress to metastatic disease after tyrosine kinase inhibition of the VEGF or mTOR pathways. Due to the positive early studies in other cancers, many groups tested the use of human antibodies against the programmed death-1 (PD-1) immune checkpoint inhibitor. Phase I studies in the metastatic RCC population demonstrated similar safety profiles, as seen in other solid tumor malignancies, with objective responses and without reaching a maximum tolerated dose (43).
A randomized Phase II trial commenced in 2014 to evaluate dose-response relationships while assessing the safety of nivolumab (Opdivo®, Bristol-Myers Squibb) in previously treated (anti-VEGF) metastatic RCC patients (excluding those with CNS or autoimmune disease) (44). There was no dose response relationship found, similar to the Phase I trial. Importantly, the median OS (18.2 to 25.5 months) was significantly higher than those of the molecular targeting agents axitinib and sorafenib (15.2 vs. 16.5 months, respectively). A subgroup analysis of PD-L1 expression and efficacy was determined after completion of the study. With a 5% PD-L1 cutoff, 27% of the patients qualified. These patients had a longer PFS (4.9 vs. 2.9 months), ORR (31% vs. 18%), and OS (NR vs. 18.2 months). If the cutoff was 1% or greater, there were no differences in the two groups.
In November of 2015, Phase III data of the CHECKMATE-025 trial provided FDA approval of nivolumab (3 mg/kg every 2 weeks) in advanced RCC patients with prior anti-VEGF therapy when compared with everolimus (Afinitor®, Novartis, 10 mg/day) (45). Since then the FDA has updated the dose of nivolumab to a flat 240 mg IV dose every 2 weeks for RCC (46). Similar patient profiles to those evaluated in the Phase II trial were incorporated into the Phase III trial. Patients had an OS of 25 months (nivolumab) versus 19.6 (everolimus) with more objective, durable responses. Importantly, adverse effects reported were less with the nivolumab cohort versus everolimus. Although overall there was no PFS difference, delayed PFS curves were seen similar to previous immunotherapy studies suggesting delayed benefit after treatment. Continuation of therapy beyond those patients with delayed responses is currently ongoing. This benefit in nivolumab was seen with or without PD-L1 expression and a subgroup analysis of those expressing greater or less than 1% PD-L1 was no different. Although patients with higher levels of PD-L1 have a poor survival, its expression is not predictive of nivolumab benefit in RCC patients (47).
Atezolizumab & avelumab: PD-L1 monoclonal antibodies
Phase I data was released in March 2016 to determine the safety and activity of atezolizumab (Tecentriq®, Roche) in 70 patients with RCC (63 clear cell, 7 non-clear cell) (48). PD-L1 expression on both the tumor cells and tumor-infiltrating immune cells was determined and scored on a 0–3 scale. Of the 63 clear cell patients, the median OS was 28.9 months with PFS of 5.6 months and with an ORR of 15%. The OS at 1 year was 81% and at 2 years 58%. Those with PD-L1 expression scored 1 or greater had an ORR of 18% and a higher 2-year survival rate while those without PD-L1 expression had an ORR of 9%. Immune related adverse effects of the class (rash) were most common and 17% of patients had Grade 3–4 fatigue, anemia, and low phosphorus. No pneumonitis or nephritis was observed. The study also looked into those who had been previously treated with an anti-VEGF TKI. Those without prior VEGF TKI therapy had a higher one and 2-year survival rate compared to previously treated patients. Interestingly, higher Fuhrman grade or sarcomatoid features had higher ORR at 22% with atezolizumab treatment. Important in this study was that VEGF refractory patients still responded, albeit in an attenuated manner versus treatment naïve patients.
Other PD-L1 inhibitors are currently being studied in combination with standard of care as well as novel TKIs. Similar to the urothelial carcinoma JAVELIN trial, the JAVELIN Renal 101 trial will investigate PD-L1 IgG1 monoclonal antibody avelumab in advanced RCC (NCT02684006). This will be a first line study of previously untreated patients using a combination of tyrosine kinase/VEGF inhibition with axitinib (Inlyta®, Pfizer) combined with avelumab. Current Phase II and III trials of atezolizumab are ongoing with and without anti-VEGF treatment modalities (bevacizumab, sunitinib, NCT02420821).
Ipilimumab: CTLA-4 monoclonal antibody
PD-1 and CTLA-4 regulate adaptive immunity in a complementary manner. Whereas CTLA-4 is upregulated early within T-Cell stimulation to taper T-Cell function, PD-1 induces T-Cell exhaustion later in the process (49). The CHECKMATE-016 trial presented Phase I data of nivolumab and ipilimumab in metastatic RCC (50,51). Combination nivolumab plus ipilimumab at various doses (nivolumab 1 mg/kg + ipilimumab 3 mg/kg versus nivolumab 3 mg/kg + ipilimumab 1 mg/kg) were given to 44 metastatic RCC patients every 3 weeks for 4 cycles followed by maintenance nivolumab at 3 mg/kg every 2 weeks. 80% of the patients were previously treated while 20% were treatment naïve. Pneumonitis, colitis, pancreatitis, and hepatitis were all seen in the initial Phase I trial with adverse effects seen in 88% of patients. ORR were between 38–45% depending on dosing profiles with 80% ongoing responses at 31–34 weeks (52). Recent data presented at ESMO 2016 revealed combination nivolumab with ipilimumab had an overall response rate of 40.4% in metastatic RCC over a 2-year follow up (50). CHECKMATE-016 continues as a Phase I trial comparing nivolumab plus various tyrosine kinase inhibitors versus nivolumab plus ipilimumab in terms of objective response rate and duration of response with an estimated completion date of 2018 (NCT01472081). Treatment adverse effects are similar to other immune checkpoint inhibitors but at an increased intensity due to the dual nature of combination inhibition.
Pembrolizumab: PD-L1 monoclonal antibody
With the success of nivolumab in the locally advanced, metastatic RCC setting, pembrolizumab trials are currently recruiting participants. The KEYNOTE-427 study is actively determining the ORR, PFS, and OS of pembrolizumab in both clear cell and non-clear cell renal carcinomas in the front line setting in treatment naïve patients. Two ongoing Phase II clinical trials are evaluating pembrolizumab with or without interferon while another is evaluating the PD-1 inhibitor with or without standard-of-care pazopanib in the metastatic RCC setting (NCT02089685, Memorial Sloan Kettering 14-074). Promising early Phase I trials of monoclonal antibody axitinib in combination with pembrolizumab (PD-1 inhibitor) or avelumab (PD-L1 inhibitor) prompted upcoming Phase III studies with these combinations (53,54). The Phase I study enrolled 52 treatment naïve RCC patients with 11 discontinuing due to progression or side effects. Of the remaining 41 patients, 35 had an objective response: 2 with CR, 33 PR, and 11 with stable disease (53).
Vaccines
Current clinical trials in metastatic RCC are isolating tumor RNA post-nephrectomy for co-electroporation with autologous dendritic cells. In addition, a synthetic CD40L RNA is added as a costimulatory molecule to permit T-Cell engagement with tumor antigens (55). A Phase II trial treating patients with nephrectomy plus sunitinib plus vaccine AGS-003 found a median PFS of 11 months and a median OS of 30 months. A Phase III trial has accrued and will determine sunitinib only versus sunitinib plus AGS-003 vaccine (55) (Figure 3).
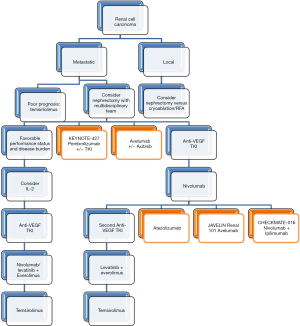
Conclusions
The integration of immunotherapy has permitted another paradigm of care for GU oncology patients. Early studies have made way for Phase III randomized clinical trials showing efficacy in a selected population of patients in need of additional therapy. These current and upcoming Phase III trials will more specifically evaluate PD-1/PD-L1 inhibition in the front-line setting for many GU malignancies. Importantly, many efficacious molecular targeting drugs are being tested in combination with PD inhibitors and the full spectrum of responses to be seen with these combos is forthcoming. Similar to results seen in melanoma with PD-1 and CTLA-4 combinations, PD-1 and anti-VEGF may also be an important synergy in RCC, while combination chemotherapy-immunotherapy in urothelial and endocrine-immunotherapy in prostate cancer may prolong OS and quality of life.
Still, there is much to be learned about immunotherapy. Specifically, there is no head-to-head data to determine if inhibition on the T-Cell side with PD-1 or the tumor side with PD-L1 has better efficacy. Upcoming trials will need to determine which provides increased tumoricidal ability and durable responses. Further, the importance of PD-L1 expression still needs to be explored. PD-L1 expression has been shown to correlate with responses in some solid tumors (non-small cell lung cancer) but not at all in others (urothelial cancer) (56). It is especially important to determine this subgroup as patients with higher PD-L1 expression may be associated with a poorer prognosis (57). Although urothelial carcinoma data shows that PD-L1 expression does not portend clinical benefit, there still may be importance in sub-selecting the patients who will respond the most to circumvent adverse effects. It remains to be understood whether PD-L1 expression is more targetable on the tumor itself or on tumor infiltrating immune cells. Next, patient stratification and selection will need to be determined so that immunotherapeutic use can be tailored to those who will respond appropriately in genitourinary malignancies. Lastly, further trials will be needed to determine the neoadjuvant use of immunotherapy before surgery, adjuvant use of immunotherapy after surgery as well as the necessity of maintenance therapy in certain patient subtypes. Immunotherapy has opened new avenues for GU oncology patients to take advantage of the innate immune system in an effort to eradicate their malignancies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Marin Feldman Xavier) for the series “Advances on Clinical Immunotherapy” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.02.09). The series “Advances on Clinical Immunotherapy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Humphrey PA. Cancers of the male reproductive organs. 2014.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- National Cancer Institute. Cancer of the Kidney and Renal Pelvis - SEER Stat Fact Sheets. 2016. Available online: https://seer.cancer.gov/statfacts/html/kidrp.html
- Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol 2010;7:245-57. [Crossref] [PubMed]
- National Cancer Institute. Bladder Cancer - SEER Stat Fact Sheets. Available online: https://seer.cancer.gov/statfacts/html/urinb.html
- Sylvester RJ. van der MEIJDEN AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol 2002;168:1964-70. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Lin Z, Xu Y, Zhang Y, et al. The prevalence and clinicopathological features of programmed death-ligand 1 (PD-L1) expression: a pooled analysis of literatures. Oncotarget 2016;7:15033-46. [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411-22. [Crossref] [PubMed]
- Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 2006;24:3089-94. [Crossref] [PubMed]
- Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 2009;115:3670-9. [Crossref] [PubMed]
- Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 2010;28:1099-105. [Crossref] [PubMed]
- Madan RA, Arlen PM, Mohebtash M, et al. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs 2009;18:1001-11. [Crossref] [PubMed]
- Small EJ, Sacks N, Nemunaitis J, et al. Granulocyte macrophage colony-stimulating factor--secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res 2007;13:3883-91. [Crossref] [PubMed]
- Drake CG. Update on prostate cancer vaccines. Cancer J 2011;17:294-9. [Crossref] [PubMed]
- Higano C, Saad F, Somer B, et al. A phase III trial of GVAX immunotherapy for prostate cancer versus docetaxel plus prednisone in asymptomatic, castration-resistant prostate cancer (CRPC). 2009 Genitourinary Cancers Symposium. Abstract: LBA150.
- Bracarda S, Altavilla A, Hamzaj A, et al. Immunologic checkpoints blockade in renal cell, prostate, and urothelial malignancies. Semin Oncol 2015;42:495-505. [Crossref] [PubMed]
- Gerritsen W R., et al. CA184-043: A randomized, multicenter, double-blind phase 3 trial comparing overall survival (OS) in patients (pts) with post-docetaxel castration-resistant prostate cancer (CRPC) and bone metastases treated with ipilimumab (ipi) vs placebo (pbo), each fol. Eur J Cancer 2013;49.
- Beer TM, Kwon ED, Drake CG, et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol 2017;35:40-47. [PubMed]
- Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol 2013;24:1813-21. [Crossref] [PubMed]
- Galsky MD, Retz M, Siefker-Radtke AO, et al. Efficacy and safety of nivolumab monotherapy in patients with metastatic urothelial cancer (mUC) who have received prior treatment: Results from the phase II CheckMate 275 study. Ann Oncol 2016;27:LBA31. _PR. [Crossref]
- Martínez-Piñeiro JA, Martínez-Piñeiro L, Solsona E, et al. Has a 3-fold decreased dose of bacillus Calmette-Guerin the same efficacy against recurrences and progression of T1G3 and Tis bladder tumors than the standard dose? Results of a prospective randomized trial. J Urol 2005;174:1242-7. [Crossref] [PubMed]
- Chang SS, Boorjian SA, Chou R, et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol 2016;196:1021-9. [Crossref] [PubMed]
- Babjuk M, Böhle A, Burger M, et al. EAU Guidelines on Non-Muscle-Invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol 2017;71:447-61. [Crossref] [PubMed]
- Shelley MD, Court JB, Kynaston H, et al. Intravesical Bacillus Calmette-Guerin in Ta and T1 Bladder Cancer. Cochrane Database Syst Rev 2000;CD001986 [PubMed]
- Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature 2013;502:333-9. [Crossref] [PubMed]
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20. [Crossref] [PubMed]
- Apolo AB, Infante JR, Hamid O, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with metastatic urothelial carcinoma from the JAVELIN solid tumor phase 1b trial: Analysis of safety, clinical activity, and PD-L1 expression. J Clin Oncol 2016;34 (suppl; abstr 4514).
- Sharma P, Bono P, Kim JW, et al. Efficacy and safety of nivolumab monotherapy in metastatic urothelial cancer (mUC): Results from the phase I/II CheckMate 032 study. J Clin Oncol 2016;34:abstr 4501.
- Bellmunt J, Sonpavde G, De Wit R, et al. KEYNOTE-045: Randomized phase 3 trial of pembrolizumab (MK-3475) versus paclitaxel, docetaxel, or vinflunine for previously treated metastatic urothelial cancer. J Clin Oncol 2015;33:abstr TPS4571.
- Balar A, Bellmunt J, O'Donnell PH, et al. Pembrolizumab (pembro) as first-line therapy for advanced/unresectable or metastatic urothelial cancer: Preliminary results from the phase 2 KEYNOTE-052 study. Ann Oncol 2016;27:LBA32. _PR. [Crossref]
- Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol 1995;13:688-96. [PubMed]
- Klapper JA, Downey SG, Smith FO, et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer 2008;113:293-301. [Crossref] [PubMed]
- McDermott DF, Cheng SC, Signoretti S, et al. The high-dose aldesleukin "select" trial: a trial to prospectively validate predictive models of response to treatment in patients with metastatic renal cell carcinoma. Clin Cancer Res 2015;21:561-8. [Crossref] [PubMed]
- Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol 2003;21:3127-32. [Crossref] [PubMed]
- Klapper JA, Downey SG, Smith FO, et al. High-dose Interleukin-2 for the Treatment of Metastatic Renal Cell Carcinoma. Cancer 2008;113:293-301. [Crossref] [PubMed]
- Figlin R, Gitlitz B, Franklin J, et al. Interleukin-2-based immunotherapy for the treatment of metastatic renal cell carcinoma: an analysis of 203 consecutively treated patients. Cancer J Sci Am 1997;3:S92-7. [PubMed]
- Schwartz RN, Stover L, Dutcher J. Managing toxicities of high-dose interleukin-2. Oncology (Williston Park) 2002;16:11-20. [PubMed]
- Anti-tumor activity of PEGylated human IL-10 (AM0010) in renal cancer alone and in combination with anti-PD1., ESMO 2016 Congress 2016:Abstract #364PD.
- Negrier S, Escudier B, Lasset C, et al. Recombinant Human Interleukin-2, Recombinant Human Interferon Alfa-2a, or Both in Metastatic Renal-Cell Carcinoma. N Engl J Med 1998;338:1272-8. [Crossref] [PubMed]
- . Interferon-alpha and survival in metastatic renal carcinoma: early results of a randomised controlled trial. Medical Research Council Renal Cancer Collaborators. Lancet 1999;353:14-7. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol 2015;33:1430-7. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
Modification of the Dosage Regimen for Nivolumab - Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 2006;66:3381-5. [Crossref] [PubMed]
- McDermott DF, Sosman JA, Sznol M, et al. Atezolizumab, an Anti-Programmed Death-Ligand 1 Antibody, in Metastatic Renal Cell Carcinoma: Long-Term Safety, Clinical Activity, and Immune Correlates From a Phase Ia Study. J Clin Oncol 2016;34:833-42. [Crossref] [PubMed]
- Curran MA, Montalvo W, Yagita H, et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 2010;107:4275-80. [Crossref] [PubMed]
- Hammers H, Plimack ER, Infante JR, et al. Updated results from a phase I study of nivolumab (Nivo) in combination with ipilimumab (Ipi) in metastatic renal cell carcinoma (mRCC): The CheckMate 016 study. Ann Oncol 2016;27:1062P. [Crossref]
- Hammers HJ, Plimack ER, Infante JR, et al. Expanded cohort results from CheckMate 016: A phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC). J Clin Oncol 2015;33:abstr 4516.
- Hammers HJ, Plimack ER, Infante JR, et al. Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC). J Clin Oncol 2014;32:5s:abstr 4504.
- Atkins MB, Plimack ER, Puzanov I, et al. Axitinib in combination with pembrolizumab in patients (pts) with advanced renal cell carcinoma (aRCC): Preliminary safety and efficacy results. Ann Oncol 2016;27:773PD.
- Motzer RJ, Choueiri TK, Larkin J, et al. 844TiP - Phase 3 study of avelumab in combination with axitinib versus sunitinib as first-line treatment for patients with advanced renal cell carcinoma (aRCC). Ann Oncol 2016;27:266-95. [Crossref]
- Amin A, Dudek AZ, Logan TF, et al. Survival with AGS-003, an autologous dendritic cell-based immunotherapy, in combination with sunitinib in unfavorable risk patients with advanced renal cell carcinoma (RCC): Phase 2 study results. J Immunother Cancer 2015;3:14. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Choueiri TK, Figueroa DJ, Fay AP, et al. Correlation of PD-L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: results from COMPARZ, a randomized controlled trial. Clin Cancer Res 2015;21:1071-7. [Crossref] [PubMed]


