
Updates in immunotherapy for acute myeloid leukemia
Introduction
The standard treatment and outcomes for adult patients with acute myeloid leukemia (AML) has not changed significantly in the past 40 years and most patients still die from their disease. The standard regimens currently used to induce remission involve high dose induction chemotherapy with cytarabine and an anthracycline. After remission is obtained, this is followed by consolidation chemotherapy to destroy residual leukemic cells. Although obtaining remission is common, the risk of relapse remains high for multifactorial reasons. AML stem cells are thought to be chemotherapy resistant and heterogeneous enough to respond to different selection pressures from chemotherapeutics (1). Additionally, AML has a multitude of techniques to evade the host immune system: Downregulation of non-self human leukocyte antigen, resist natural killer (NK) cells, decreasing antigenicity, producing their own dendritic cells (DCs) leading to T-cell neutralization, and releasing ligands to block T cell attacks (2). For those at increased risk, the best current curative approach is with an allogeneic hematopoietic stem cell transplant (aHSCT). Those who are medically fit to tolerate an aHSCT then have a natural immunotherapy at their defense: graft-versus-leukemic (GVL) effect, where donor NK and T cells are able to destroy both leukemic and leukemic stem cells. Unfortunately, relapse after aHSCT occurs, and the treatment has high morbidity and mortality rates due to increased infection risk, graft-versus-host disease, and additional cancers. A method of utilizing immunotherapy to destroy leukemic cells without the morbidity of a stem cell transplant has garnered much interest over the past several decades. The antitumor effects of immunotherapy have also been recognized in methods other than aHSCT. For example, although the primary mechanism of action for anthracyclines is cytotoxicity, they have shown to stimulate lymphocytes and in animal models success of therapy correlates with the functional status of the immune system (3,4). A search for further methods using the strategies noted above to produce a prolonged remission state with minimal toxicity and risk factors is currently underway.
Four methods of novel immunotherapies will be discussed in this review: monoclonal antibodies (MoAbs) and immuno-chemotherapy, vaccinations against AML antigens, chimeric antigen receptor T-cells (CAR T-cells), and checkpoint inhibitors.
Vaccination
Vaccination is a method of immunotherapy that can actively stimulate a patient’s immune system to recognize and destroy AML through introduction of a tumor antigen. Vaccines against AML were first created in the late 1960s, combining the tuberculosis peptide bacille Calmette-Guérin antigen and irradiated AML cells to stimulate the immune system as maintenance therapy. Unfortunately, this method failed to produce any clinical benefit in three of the four randomized control trials; although one smaller trial did note increased survival and remission duration of vaccination maintenance immunotherapy after induction and consolidation compared to observation, these results were not observed in larger clinical trials. Additionally, this study was limited by insufficient induction and consolidation treatments compared to today’s standard, limiting usefulness and relevance of conclusions (5,6).
Currently, vaccines are constructed from intact AML cells that are inactivated via radiation, leukemia-associated antigens (LAA) (Table 1), which are antigens, associated with a tumor type but may be present on normal cell lines, or through leukemia-specific antigens (LSA), which are antigens specific to a tumor. These vaccinations would be given in conjunction with standard chemotherapeutic regimens in hope that they could prevent relapse through targeting cells that are chemo-refractory, such as stem cells. LSAs are preferred targets to prevent inadvertent destruction of normal myeloid cell lines, but may be expressed in only a minority of AML types (e.g., PML-RARA). LAAs are more common but less specific, potentially inducing systemic toxicity to normal myeloid lines. If these antigens are more highly expressed on an AML cell compared to healthy cells, however, there is potential benefit in its use as a target (9).
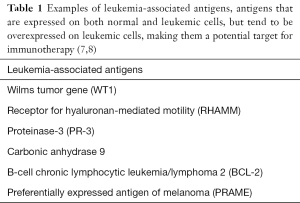
Full table
We will discuss two methods of vaccination strategies, DC vaccination and peptide vaccinations.
DC vaccination
DCs are antigen presenting cells (APCs), matured from circulating monocytes. Vaccine utilization of these specific cells has gathered great interest due to the strong immune response they produce. These AML-specific DCs are produced by host monocytes that undergo in vitro differentiation to DCs with granulocyte macrophage colony stimulating factor and interleukin-4, then primed by introduction of LAAs through mRNA electroporation before injected (9). These cells possess the capability of presenting antigens to cytotoxic T lymphocytes (CD8+ T cells) in vivo, inducing immune response (8).
A common method of creating DC vaccinations is with Wilms tumor 1 (WT1), an oncogenic protein that is associated with lower survival rates and overexpressed in AML (7,10). In 2013, a phase II clinical trial by Berneman et al. utilized DCs produced from monocytes electroporated with WT1 mRNA as a vaccination for 29 patients with AML at high risk of relapse, 26 of whom were in complete remission (three in partial remission), trialing three separate constructs for the vaccine. The most effective method appeared to be construct 1, which encoded full-length WT1. This resulted in a molecular response (normalization of elevated WT1 transcript levels) in 7 of 13 patients trialed; 8 patients in total responded. Of these 8, 5 remained in complete remission, and 4 are maintaining remission 5 years from treatment and considered cured. One patient in partial remission achieved complete remission after vaccination (11). Given these preliminary outcomes, a phase II clinical trial for patients over 65 is underway (11,12).
Recently, a new vaccine based on telomerase-focused DCs, AST-VAC1, successfully underwent a phase II trial in 2015. The vaccine was tolerated well other than one case of ITP, headaches and fatigue. Eleven of the 19 patients in complete remission remained disease free after 52 months of follow up, including four of seven patients older than 60 (13).
Peptide vaccinations
Peptide vaccinations have been researched heavily with overall disappointing results in AML (7,9,12). Utilizing receptor for hyaluronic acid mediated motility (RHAMM), PR3, or WT-1 peptides the vaccinations were found to be immediately immunogenic, but without lasting response and clinical improvement despite repeated vaccinations (12). One study hypothesized this may be due in part to the activation of cytotoxic T cells without activation of memory CD4 T cells (14). This recent phase I study in 2015 involving recruitment of CD4s and CD8s simultaneously as targets of activation via class I and II peptide epitopes found promising results in 14 patients with AML in first (CR1) or second remission (CR2). This method was generally well tolerated and CR2 had increased survival with 495 days compared to historical controls; however, all patients ultimately had disease progression, and the authors noted a lack of a robust detectible immune response, suggesting that peptide vaccinations may not be sufficient to achieve long-term immunity alone (14).
MoAbs and immuno-chemotherapy
MoAbs are lab-created versions of antibodies that search for specific targets to aid in destruction. There are multiple mechanisms for antibody mediated destruction, with some involving the innate immune system through increased identification and elimination by T cells, or by attaching chemotherapeutic agents to the antibody of choice (immuno-chemotherapy). There has been significant success in acute lymphocytic leukemia (ALL) due to the CD-19 cell marker, with multiple medications in clinical trials with promising results (15).
Compared with ALL, AML has had less significant breakthroughs in MoAbs, partially due to difficulty in identifying an ideal target. Identification of an ideal AML target is limited due to the fact that many candidate antigens are also found on healthy myeloid precursors with potential on target off-tumor effects of aplasia or neutropenia. Targets currently being investigated for AML include CD33, CD47, and CD123. An anti-CD33 antibody conjugated to an antitumor antibiotic calicheamicin, gemtuzumab ozogamacin (GO, also known as Mylotarg), had received accelerated approval in 2000 by the United States Food and Drug Administration for use in first relapse AML in patients unable to receive systemic chemotherapy. Unfortunately, after significant toxicity and lack of improvement in clinical outcomes during a phase 3 study (S0106), GO was removed from the market in 2010 (16). However, upon further review of S0106, there was noted to be additional factors that could have accounted for the results, such as abnormally low induction mortality in control group, inappropriately high dosage of GO, and inadequately dosed chemotherapeutic regimens in the experimental group (9,16). Further studies are now being conducted to revisit GO as a potential therapy. A Blood article in April 2013 outlined several studies that have shown promising results. To summarize four large studies conducted utilizing GO along with current chemotherapeutic regimens, event-free survival was found to be improved in populations with favorable cytogenetics and favorable/intermediate risk groups, along with some benefit in overall survival in several of the studies. However, there was no benefit in the unfavorable group. Hepatotoxicity was also found to be reduced in the lower doses utilized in current studies compared to prior investigations (16). These promising results are now leading to phase 4 clinical trials for patients with relapsed disease (17).
Another anti-CD33 MoAB is also being developed (9,18). Vadastuximab talirine (SGN-CD33A) is an anti-CD33 antibody conjugated with pyrrolobenzodiazepine (PBD), a DNA-binding agent. The chemotherapeutic agent is internalized after interaction with the anti-CD33 MoAB to destroy AML cells. The novel drug is currently in phase 3 clinical trials as of August 2016 (CASCADE trial). In this trial, vadastuximab is being combined with azacitidine or decitabine for older adults with newly diagnosed AML. Additional phase I and II trials are also being evaluated for relapsed AML, pre-treatment for aHSCT, and myelodysplastic syndrome (18). While GO showed promise with favorable cytogenetics and favorable or intermediate risk groups, vadastuximab appears to be efficacious in poor-risk cytogenetics in ongoing research (19).
Another CD marker for antibody targeting that has been recognized is CD123, present on all myeloid cell lines but highly expressed in AML (20). An anti-CD123 antibody with a specific Fc-domain modification to increase NK cell binding, CSL362, was found to have in vivo efficacy of delaying leukemic progression with and without NK cell infusion for immunosuppressed mice following chemotherapy. It was noted during treatment that CD123 would be downregulated, consistent with AML ability to evade the immune system; however, the cell marker would return 25 days later. CSL362 recently completed a phase I clinical trial and is now recruiting for phase II clinical trial in conjunction with decitabine (21,22).
CAR T-cells
Chimeric antigen receptors (CARs) are engineered to include an antigen recognition domain with one or more intra-cellular signaling domains. Through gene transfer techniques, CARs can then be introduced ex-vivo into T cells, re-directing them to target the desired tumor antigen. Autologous anti-CD19 CAR T-cells have been remarkably successful inducing remissions in patients with relapsed and refractory CD19 positive B cell malignancies including CLL, ALL and Non-Hodgkin’s lymphoma (23-26).
The challenges with CAR T-cells and AML are similar to that of MoAbs. CD19 is an ideal target in that off-tumor CD19 expression is limited only to mature B cells. This results in predicted on-target off-tumor side effect of B cell aplasia and resulting hypogammaglobulinemia but this is manageable with IVIG replacement therapy and is not associated with significant risk. Unfortunately finding an ideal target for AML is more challenging due to expression of potential targets on healthy myeloid precursors (27). However, there has been some intriguing studies, with the potential for further research to come.
In 2013, Ritchie et al. were able to successfully develop CAR T-cells against one of the over-expressed antigens in AML lines, called LeY (27). These antigens are actually defucosylated carbohydrates, and T-cells were found to expand and persist for up to 10 months upon infusion of 5 patients with relapsed AML and LeY positivity. Although all patients relapsed, two of four patients had reduction in disease. Unlike some antigens present on AML cells which will decrease expression when targeted by the immune system and decrease immune response, LeY was found to not downregulate, indicating that it could be a potential future target. CAR T-cells were also found to reach bone marrow and skin, and thus would be able to impact leukemic cells throughout the body, both peripherally and sequestered in bone marrow. It was ultimately concluded that CAR T-cells alone using this method was insufficient to control AML, and perhaps usage of checkpoint inhibitors may be beneficial to prevent inactivation (27).
Other targets have been the same as those noted above for MoAbs. A clinical trial in 2015 found significant disease response using CD33 as a target for CAR T-cells in a patient with multiple-relapsed AML. Unfortunately, this led to significant adverse reactions during and after infusion, such as fevers and chills, elevated cytokine levels, fluctuating pancytopenia and hyperbilirubinemia. Although disease response was observed with decreased blasts two weeks after treatment, the patient ultimately progressed nine weeks later (28).
In a mouse model, AML targeting with anti-CD123 CAR T-cells (CART123) resulted in a potent inflammatory response with clear antitumor activity and increased survival (29). CART123 response correlated with CD123 increased expression, and CART123 was found to have persistence, with more rapid destruction of AML cells in leukemia re-challenged mice, a significant improvement from prior monoclonal antibody trials. However, hematopoietic off-tumor toxicity was observed with aplasia, suggesting that this regimen may be intolerable without an aHSCT rescue strategy especially if persistence of anti-CD123 CAR T-cells is anticipated (29).
Checkpoint inhibitors
Immune checkpoints are methods inherent to the immune system to prevent autoimmunity from occurring. In normal circumstances, the checkpoint molecules (Table 2) are responsible to prevent cross-reactivity with self via T-cell receptor ligand binding and resultant inhibition of T-cell function (29).
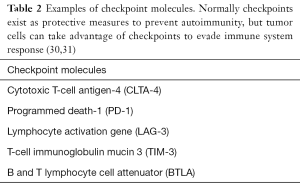
Full table
Tumors take advantage of these checkpoints by expressing PD-L1 (ligands for PD-1+ cells) thereby inducing T-cell dysfunction, or by increasing indoleamine 2,3-dioxygenase (IDO), an enzyme which catabolizes tryptophan leading to regulatory T cell formation (Tregs) (32,33). Notably this has been associated with poorer prognosis in multiple malignancies (31,34) It has been noted that long-term overexpression of these checkpoint molecules can reduce anti-tumor effect from therapy, and that these tend to be upregulated with cytokines, including interferon-gamma (32).
Therapies targeted to overcoming these mechanisms have revolutionized treatment of solid tumors such as metastatic melanoma with ipilimumab and pembrolizumab (35,36), and metastatic non-small cell lung cancer with recent FDA approval of atezolizumab, a programmed cell death ligand-1 (PD-L1) inhibitor, in October 2016 (37). In AML specifically, there has been some conflicting evidence regarding PD-1 overexpression and if it is a byproduct of proliferating T-cells at relapse as opposed to a process intrinsic to AML (38). There has also been the question of whether it has clinical relevance, especially in treatment-naïve disease (31,38,39).
In terms of current clinical trials for AML, there has been some promise in utilization of PD-1 inhibitors in conjunction with AMG 330, a CD33/CD3-directed bispecific T cell engaging (BiTE) antibody. In this study, increased immune response was observed when combination was used with increased lysis of tumor cells, increased T cell proliferation, and upregulation of PD-L1 with cytokines (IFN-gamma and TNF-alpha) (40). A clinical trial involving nivolumab, a PD-1 inhibitor, is currently recruiting for phase II clinical trials evaluating efficacy of eliminating minimal residual disease and relapse prevention (41). There are further phase I/II trials utilizing nivolumab in conjunction with idarubicin and cytarabine in both AML and myelodysplastic syndrome (42), a phase II trial using in high risk patients (43), and nivolumab independently or with ipilimumab (CTLA-4 antibody) for potential benefit after aHSCT (44).
Conclusions
It is clear that the immune system plays an important role in preventing and controlling AML supporting an immunotherapeutic approach to this disease. The tremendous success of check point inhibitors in solid tumors may have similar impact in treating AML and clinical trials to address this question are ongoing. Challenges unique to exploiting targeted antibody and CAR T cell therapy for this disease result from the potential for significant hematopoietic toxicity as many potential target antigens are also expressed on myeloid precursors. This is particularly a challenge with CAR T-cells which have the potential for amplified off tumor on target effects with toxicity potentially linked to the persistence of the agent. Different approaches to overcome these limitations are being explored in pre-clinical and clinical trials.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Advances on Clinical Immunotherapy”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.01.14). The series “Advances on Clinical Immunotherapy” was commissioned by the editorial office without any funding or sponsorship. MFX served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Translational Cancer Research. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Horton SJ, Huntly BJ. Recent advances in acute myeloid leukemia stem cell biology. Haematologica 2012;97:966-74. [Crossref] [PubMed]
- Teague RM, Kline J. Immune evasion in acute myeloid leukemia: current concepts and future directions. J Immunother Cancer 2013;1. pii: 1/1/13.
- Zhang Z, Yu X, Wang Z, et al. Anthracyclines potentiate anti-tumor immunity: A new opportunity for chemoimmunotherapy. Cancer Lett 2015;369:331-5. [Crossref] [PubMed]
- Mattarollo SR, Loi S, Duret H, et al. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res 2011;71:4809-20. [Crossref] [PubMed]
- Rashidi A, Walter RB, Tallman MS, et al. Maintenance therapy in acute myeloid leukemia: an evidence-based review of randomized trials. Blood 2016;128:763-73. [Crossref] [PubMed]
- Omura GA, Vogler WR, Lefante J, et al. Treatment of acute myelogenous leukemia: influence of three induction regimens and maintenance with chemotherapy or BCG immunotherapy. Cancer 1982;49:1530-6. [Crossref] [PubMed]
- Lin C, Li Y. The role of peptide and DNA vaccines in myeloid leukemia immunotherapy. Cancer Cell Int 2013;13:13. [Crossref] [PubMed]
- Schmitt M, Casalegno-Garduño R, Xu X, et al. Peptide vaccines for patients with acute myeloid leukemia. Expert Rev Vaccines 2009;8:1415-25. [Crossref] [PubMed]
- Lichtenegger FS, Schnorfeil FM, Hiddemann W, et al. Current strategies in immunotherapy for acute myeloid leukemia. Immunotherapy 2013;5:63-78. [Crossref] [PubMed]
- King-Underwood L, Pritchard-Jones K. Wilms' tumor (WT1) gene mutations occur mainly in acute myeloid leukemia and may confer drug resistance. Blood 1998;91:2961-8. [PubMed]
- Berneman ZN, Van de Velde A, Anguille S, et al. Prevention of relapse in acute myeloid leukemia by dendritic cell vaccination: report on a phase II study with 29 patients. Blood 2013;122:236.
- Lichtenegger FS, Krupka C, Köhnke T, et al. Immunotherapy for Acute Myeloid Leukemia. Semin Hematol 2015;52:207-14. [Crossref] [PubMed]
- AML Vaccine Proves Feasible, Potentially Beneficial | Cancer Network. Cancernetwork.com. 2016 [cited 31 October 2016]. Available online: http://www.cancernetwork.com/asco-2015-hematology/aml-vaccine-proves-feasible-potentially-beneficial
- Brayer J, Lancet JE, Powers J, et al. WT1 vaccination in AML and MDS: A pilot trial with synthetic analog peptides. Am J Hematol 2015;90:602-7. [Crossref] [PubMed]
- Naddafi F, Davami F. Anti-CD19 Monoclonal Antibodies: a New Approach to Lymphoma Therapy. Int J Mol Cell Med 2015;4:143-51. [PubMed]
- Rowe JM, Löwenberg B. Gemtuzumab ozogamicin in acute myeloid leukemia: a remarkable saga about an active drug. Blood 2013;121:4838-41. [Crossref] [PubMed]
- Expanded Access/Compassionate Use Protocol For Relapsed Or Refractory CD33 Positive AML Patients In The USA Without Access To Comparable Or Alternative Therapy - Full Text View - ClinicalTrials.gov. Clinicaltrials.gov. 2016 [cited 30 October 2016]. Available online: https://clinicaltrials.gov/ct2/show/NCT02312037
- Phase 3 trial of 33A initiated for patients with newly diagnosed AML - CD33A. Adc-cd33.com. 2016 [cited 30 October 2016]. Available online: http://adc-cd33.com/announcements/phase-3-trial-of-33a-initiated-for-patients-with-newly-diagnosed-aml/
- Bixby DL, Stein AS, Fathi AT, et al. Vadastuximab Talirine Monotherapy in Older Patients with Treatment Naive CD33-Positive Acute Myeloid Leukemia (AML). Blood 2016;128:590.
- Lee EM, Yee D, Busfield SJ, et al. Efficacy of an Fc-modified anti-CD123 antibody (CSL362) combined with chemotherapy in xenograft models of acute myelogenous leukemia in immunodeficient mice. Haematologica 2015;100:914-26. [Crossref] [PubMed]
- A Study of CSL362 in Patients With CD123+ Acute Myeloid Leukemia Currently in Remission ClinicalTrials.gov. Clinicaltrials.gov. 2016 [cited 31 October 2016]. Available online: https://clinicaltrials.gov/ct2/show/NCT01632852
- An Efficacy and Safety Study of Decitabine (DACOGEN) Plus JNJ-56022473 (Anti CD123) Versus Decitabine (DACOGEN) Alone in Participants With Acute Myeloid Leukemia (AML) Ineligible for Intensive Chemotherapy - ClinicalTrials.gov. Clinicaltrials.gov. 2016 [cited 31 October 2016]. Available online: https://clinicaltrials.gov/ct2/show/NCT02472145
- Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224ra25 [Crossref] [PubMed]
- Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015;7:303ra139 [Crossref] [PubMed]
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015;385:517-28. [Crossref] [PubMed]
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507-17. [Crossref] [PubMed]
- Brenner MK. CAR T cells for acute myeloid leukemia: the LeY of the land. Mol Ther 2013;21:1983-4. [Crossref] [PubMed]
- Wang QS, Wang Y, Lv HY, et al. Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol Ther 2015;23:184-91. [Crossref] [PubMed]
- Gill S, Tasian SK, Ruella M, et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood 2014;123:2343-54. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Sehgal A, Whiteside TL, Boyiadzis M. Programmed death-1 checkpoint blockade in acute myeloid leukemia. Expert Opin Biol Ther 2015;15:1191-203. [Crossref] [PubMed]
- Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res 2012;72:5435-40. [Crossref] [PubMed]
- New Approaches for the Immunotherapy of Acute Myeloid Leukemia. Discoverymedicine.com. 2016 [cited 30 October 2016]. Available online: http://www.discoverymedicine.com/Terrence-L-Geiger/2015/04/new-approaches-for-the-immunotherapy-of-acute-myeloid-leukemia/
- Folgiero V, Goffredo BM, Filippini P, et al. Indoleamine 2,3-dioxygenase 1 (IDO1) activity in leukemia blasts correlates with poor outcome in childhood acute myeloid leukemia. Oncotarget 2014;5:2052-64. [Crossref] [PubMed]
- Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res 2011;17:6958-62. [Crossref] [PubMed]
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521-32. [Crossref] [PubMed]
- Lowes R. Tecentriq Approved for Metastatic Non-Small Cell Lung Cancer. Medscape 2016 [cited 14 November 2016]. Available online: http://www.medscape.com/viewarticle/870495
- Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood 2009;114:1545-52. [Crossref] [PubMed]
- Schnorfeil FM, Lichtenegger FS, Emmerig K, et al. T cells are functionally not impaired in AML: increased PD-1 expression is only seen at time of relapse and correlates with a shift towards the memory T cell compartment. J Hematol Oncol 2015;8:93. [Crossref] [PubMed]
- Krupka C, Kufer P, Kischel R, et al. Blockade of the PD-1/PD-L1 axis augments lysis of AML cells by the CD33/CD3 BiTE antibody construct AMG 330: reversing a T-cell-induced immune escape mechanism. Leukemia 2016;30:484-91. [Crossref] [PubMed]
- Nivolumab in Eliminating Minimal Residual Disease and Preventing Relapse in Patients With Acute Myeloid Leukemia in Remission After Chemotherapy - Full Text View - ClinicalTrials.gov. Clinicaltrials.gov. 2017 [cited 13 November 2016]. Available online: https://clinicaltrials.gov/ct2/show/NCT02275533
- Study of Idarubicin, Cytarabine, and Nivolumab in Patients With High-Risk Myelodysplastic Syndrome (MDS) and Acute Myeloid Leukemia (AML) - Full Text View - ClinicalTrials.gov. Clinicaltrials.gov. 2017 [cited 16 January 2017]. Available online: https://clinicaltrials.gov/ct2/show/NCT02464657
- Nivolumab in Acute Myeloid Leukemia (AML) in Remission at High Risk for Relapse - Full Text View - ClinicalTrials.gov [Internet]. Clinicaltrials.gov. 2017 [cited 17 January 2017]. Available online: https://clinicaltrials.gov/ct2/show/NCT02532231
- Single Agent and Combined Inhibition After Allogeneic Stem Cell Transplant - Full Text View - ClinicalTrials.gov [Internet]. Clinicaltrials.gov. 2017 [cited 17 January 2017]. Available online: https://clinicaltrials.gov/ct2/show/NCT02846376


