
Moving the mountain in advanced non-small-cell lung cancer: evolving immunotherapies for a dire disease
Despite advances in diagnostic, surgical/interventional, and supportive care strategies, lung cancer remains a lethal entity representing the most common cause of cancer-related mortality worldwide (1). Non-small cell lung cancers (NSCLCs) represent the vast majority of these cases. With more than half of all patients presenting with advanced stage disease at initial diagnosis, there has been a persistent and pressing need for improved systemic therapies—both with regards to efficacy and toxicity. Even so, platinum doublets have remained the mainstay of palliative therapy for the past several decades. Based on a number of randomized trials, platinum doublet chemotherapy administered to fit and willing patients achieves improved survival and quality of life (QoL) as compared to best supportive care alone and has long remained the unchallenged standard of care (2). Though many chemotherapeutic agents have been studied in combination with a platinum agent, none has demonstrated superior outcomes in unselected cohorts (3).
In recent years, there have been key developments in our understanding of this heterogeneous disease, with growing appreciation for the impact of tumor-specific histopathology and molecular characterization on the clinical course and response to various systemic therapies. Specifically, this includes demonstration of a survival benefit in patients with nonsquamous histology receiving the antimetabolite pemetrexed as part of the platinum doublet (4), pemetrexed maintenance therapy in patients with adenocarcinoma histology and stable disease/treatment response following four to six cycles of first line platinum doublet therapy (5), and addition of bevacizumab to platinum doublet in patients with nonsquamous disease (6).
The recognition and characterization of molecularly defined subsets of patients with oncogene-addicted advanced NSCLC and actionable therapeutic targets has further transformed the landscape of this disease. Identification of oncogenic driver mutations or gene rearrangements in the epidermal growth factor receptor (EGFR) (10–15% of advanced NSCLC), anaplastic lymphoma kinase (ALK) (3–5% of advanced NSCLC), and ROS proto-oncogene 1 (ROS1) (1–2% of advanced NSCLC) and application of precision tyrosine kinase inhibitors (TKIs) have rendered the ability to optimally match targeted systemic therapies with tumor-specific abnormalities—particularly in lung adenocarcinomas.
To date, seven oral targeted therapies have been approved by the United States Food and Drug Administration (FDA) for use in molecularly defined subsets of advanced NSCLC: erlotinib, gefitinib, and afatinib for tumors with sensitizing EGFR mutations; osimertinib for tumors with the EGFR T790M mutation; crizotinib, ceritinib, and alectinib for tumors with ALK gene rearrangements; and crizotinib for tumors with ROS1 gene rearrangements. Across multiple randomized studies comparing these TKIs with conventional cytotoxic chemotherapy, a consistent theme has emerged: brisk [objective response rates (ORRs) on the order of 60–80%] and durable improvements in clinical outcomes [progression-free survival (PFS) on the order of 9–12 months] with lesser toxicity and better QoL as compared to chemotherapy (7-14). Thus, since 2013, expert guidelines have recommended routine testing for EGFR mutations and ALK gene rearrangements on all tumor specimens for patients with advanced NSCLC and an adenocarcinoma component (or inability to exclude adenocarcinoma)—regardless of clinical, demographic, or other characteristics (15).
Taken together, the standard of care for management of advanced NSCLC in recent years has emphasized upfront stratification in medically fit patients on the basis of: (I) actionable molecular targets (i.e., EGFR mutations or ALK/ROS1 gene rearrangements) and (II) histology (i.e., nonsquamous vs. squamous). In patients with an identified actionable molecular target, the use of an upfront oral palliative TKI is the evidence-based standard. For those patients with no actionable molecular target, first line intravenous (IV) palliative chemotherapy with a platinum doublet is recommended; addition of bevacizumab and maintenance chemotherapy are added considerations in these patients (Figure 1).
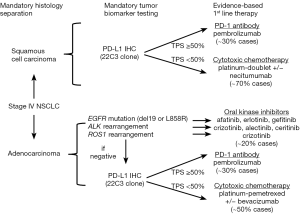
Even despite such advances, however, the median overall survival (OS) for advanced NSCLC treated with palliative chemotherapy has not been moved beyond 9–12 months. Further, availability of an actionable, FDA-approved targeted therapy will only be relevant in some 20–25% of all patients with advanced NSCLC—and primarily in patients with adenocarcinoma histology. More tailored paradigms for management of squamous cell lung cancers is an area of unmet need, as use of pemetrexed, bevacizumab, or oral TKIs is generally not indicated/relevant in this tumor histology. Thus, moving beyond conventional chemotherapy to identify more broadly applicable, durably efficacious, and less toxic systemic therapies has remained a dire unmet need in advanced NSCLC—perhaps until now.
Immune checkpoint inhibitors have afforded a novel approach to antineoplastic therapy. By impeding inhibitory signals affecting cancer-targeting T lymphocytes, the host anticancer immune response is reignited. Monoclonal antibodies inhibiting both programmed death 1 (PD-1) (nivolumab and pembrolizumab) and programmed death ligand 1 (PD-L1) (atezolizumab) have demonstrated significant promise in the management of advanced NSCLC. Notable and durable responses were observed in the early phase trials of these drugs in heavily pretreated, treatment-refractory patients with advanced NSCLC (16). Subsequent large randomized studies have demonstrated the superiority of the immune checkpoint inhibitors nivolumab, pembrolizumab, and atezolizumab as compared to palliative docetaxel in the second line setting with regards to OS, magnitude and durability of response, and treatment-related toxicity (17-20). Since October 2015, three immune checkpoint inhibitors (nivolumab, pembrolizumab, and atezolizumab) have garnered FDA approval for use in advanced NSCLC without actionable EGFR/ALK aberrations in the first (pembrolizumab) or second (nivolumab, pembrolizumab, and atezolizumab) line settings—regardless of squamous/nonsquamous histology.
Identifying determinants of therapeutic benefit by way of predictive biomarkers has been an ongoing era of investigation and debate. PD-L1 status—either on tumor cells, tumor-infiltrating immune cells, or both—has been the major emphasis. However, clinical trials of PD-1 and PD-L1 inhibitors in advanced NSCLC to date have shown conflicting results with regard to the predictive impact of PD-L1 immunohistochemistry (IHC). Definitions of PD-L1 “positivity” (i.e., staining of tumor cells vs. tumor-infiltrating immune cells or both and quantitative thresholds) have varied considerably across studies as have methods of PD-L1 testing (i.e., different diagnostic antibodies, scoring systems, and technical platforms). Not unsurprisingly, therefore, correlation between biomarker positivity and treatment response rates has varied widely (13–83% depending on the study in question) (21). Moreover, rates of therapeutic response in patients deemed PD-L1 IHC “negative” have not been insignificant (3–20%)—especially given that responses to second line palliative docetaxel have historically been on the order of ≤10% and with far greater toxicity than seen with immune checkpoint inhibitors (21). To date, only pembrolizumab has acquired an FDA-approved companion diagnostic, the PD-L1 IHC 22C3 pharmDx assay (Dako North America, Inc.). Further, it is the only one of the immune checkpoint inhibitors that has been FDA approved in advanced NSCLC for use selectively in patients with PD-L1 positive tumors—though thresholds for PD-L1 tumor proportion score (TPS) “positivity” are defined differently in the first line (PD-L1 TPS ≥50%) vs. second line (PD-L1 TPS ≥1%) settings.
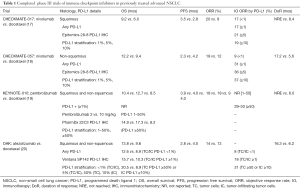
Findings from the four major phase III randomized trials of the FDA-approved immune checkpoint inhibitors for previously treated advanced NSCLC are summarized in Table 1.
Full table
It is amidst this burgeoning landscape that Reck and colleagues published KEYNOTE-024, a phase III randomized study of first line pembrolizumab vs. platinum doublet for previously untreated, PD-L1 positive (TPS ≥50%) stage IV NSCLC (22). In this study, 305 patients were randomly assigned to either pembrolizumab administered IV at a flat dose of 200 mg every 3 weeks for 35 weeks or platinum doublet (carboplatin/pemetrexed, cisplatin/pemetrexed, carboplatin/gemcitabine, cisplatin/gemcitabine, or carboplatin/paclitaxel at the investigator’s discretion) given IV every 3 weeks for four to six cycles. The most common regimen for the group randomized to conventional chemotherapy was pemetrexed (44.4%), more than half of whom went on to receive pemetrexed maintenance. The majority of patients were male, current/former tobacco users, and with nonsquamous histology. Tumor PD-L1 IHC was assessed using the FDA-approved companion diagnostic (pharmDx 22C3) and was performed on core/excisional biopsies obtained at the time that metastatic disease was diagnosed; fine needle aspirates or archival specimens obtained from sites treated with any intervening radiation therapy or chemotherapy were not permitted. Of 1,653 patients whose samples were evaluable for PD-L1, 30.2% had a PD-L1 TPS of ≥50%, thus meeting the threshold for positivity for entry into the trial.
With a median follow-up of 11.2 months, the primary endpoint of PFS in the pembrolizumab vs. chemotherapy arms was a significant 10.3 vs. 6.0 months [hazard ratio (HR) for disease progression/death =0.50, P<0.001]. The estimated rate of OS at 6 months was also increased in the pembrolizumab vs. chemotherapy group (80.2% vs. 72.4%, P=0.005). Response rates were 44.8% vs. 27.8% in the pembrolizumab vs. chemotherapy groups, respectively, consistent with response rates reported for platinum doublet therapy in this setting previously in the literature. Further, median duration of response (DoR) was notably longer in the pembrolizumab vs. chemotherapy group: not reached vs. 6.3 months, respectively. Crossover from chemotherapy to pembrolizumab was allowed, and 43.7% of patients initially receiving chemotherapy subsequently crossed over to the immunotherapy arm. Additionally, the study was stopped early at the recommendation of the external data and safety monitoring committee due to evidence of superior OS with pembrolizumab vs. chemotherapy, thus allowing patients receiving chemotherapy the opportunity to receive pembrolizumab.
The toxicity profile noted with pembrolizumab was consistent with previous reports of PD-1/PD-L1 antibodies and favorable as compared with chemotherapy: grade 3–5 treatment-related adverse events (AEs) were 26.6% vs. 53.3%, respectively. The most common treatment-related AEs in the pembrolizumab group were diarrhea (14.3%), fatigue (10.4%), and pyrexia (10.4%). Immune-mediated AEs were noted in 29.2% of patients receiving pembrolizumab; however, grade 3–4 immune-related AEs occurred infrequently and included: severe skin reactions (3.9%), pneumonitis (2.6%), and colitis (1.3%).
Notably, preliminary results have also recently been reported for CheckMate-026, a phase III study of nivolumab vs. platinum doublet chemotherapy in patients with previously untreated, PD-L1 positive (defined as present in ≥1% of tumor cells) advanced NSCLC (23). A total of 541 patients were randomized in a 1:1 fashion to receive weight-based nivolumab 3 mg/kg IV every 3 weeks or investigator’s choice of platinum doublet chemotherapy (same as in KEYNOTE-024) IV every 3 weeks for up to six cycles. Patients progressing on chemotherapy were allowed to crossover to nivolumab. OS was 14.4 vs. 15.2 months for nivolumab vs. chemotherapy (HR 1.02). The primary endpoint of improved PFS in patients whose tumors were “strongly” PD-L1 positive (i.e., PD-L1 ≥5% by IHC) was not met. No new safety signals were observed with nivolumab, and serious AEs were seen in 18% vs. 51% of patients receiving nivolumab vs. chemotherapy, respectively.
The conflicting outcomes of these two rigorously conducted phase III studies of frontline immunotherapy in advanced NSCLC have raised important questions regarding optimal patient selection and perpetuate the controversies pertaining to PD-L1 as a predictive biomarker. To date, there have been no head to head comparisons of the various PD-1 or PD-L1 targeting agents, though we have generally considered that they are equally efficacious. Though PD-L1 positivity has in numerous studies now been associated with improved response rates and survival outcomes, multiple questions persist. Were different thresholds for defining PD-L1 “positivity” (i.e., TPS ≥50% in KEYNOTE-024 vs. ≥1% in CheckMate-026) enough to explain these divergent outcomes? How much PD-L1 is “enough”? What is the optimal method for assessing PD-L1 status—tumor cells, tumor-infiltrating immune cells, both? What is the optimal platform for PD-L1 testing? How should the existing platforms best be harmonized? Addressing the latter issue has become an important priority as this therapeutic domain has evolved. Initial results from the Blueprint PD-L1 IHC Assay Comparison Project suggest that 3 of the 4 most commonly utilized PD-L1 IHC assays in the key trials of immune checkpoint inhibitors in NSCLC to date [22C3 (pembrolizumab), 28-8 (nivolumab), and SP263 PD-L1 IHC as opposed to the SP142 assay (atezolizumab)] demonstrate PD-L1 expression to a similar degree, though interchanging assays and cut-offs may still lead to “misclassification” of PD-L1 status in some cases (24).
In sum, the experience of immune checkpoint inhibitors in the care of patients with advanced NSCLC has given credence to some recurring themes: (I) ORRs are generally in the 10–30% range, regardless of PD-L1 status (though patients whose specimens express higher PD-L1 may experience a greater likelihood of response and long-term outcomes); (II) in those patients achieving a response, the response is often durable (i.e., lasting many months and often superseding the more limited DoR seen with conventional chemotherapy); and (III) toxicity profiles with the immunotherapeutic agents are generally less severe than those historically seen with conventional chemotherapy—though the identification and management of immune-mediated AEs requires heightened awareness on the part of patients and providers alike to permit early intervention.
In the second line setting and beyond, conventional chemotherapy has proven inferior to immune checkpoint inhibitors both with regards to outcomes and toxicity—regardless of PD-L1 status and other patient selection factors—in patients who are otherwise deemed fit to continue with cancer-directed therapy. This reflects the hugely unsatisfying outcomes for patients with this difficult disease and the heretofore modest options available to patients whose disease has progressed on first line platinum-based therapy. With the approval of EGFR-(~10–15%), ALK- (~3–5%), and ROS1- (~1–2%) targeting TKIs and pembrolizumab (~30%) in defined subsets of patients, some 50% of patients with advanced NSCLC will now have an option for a frontline, tumor-specific systemic palliative therapy (25). Additional needed exploration is ongoing to see if combining immunotherapies (either with themselves or concurrently/sequentially with chemotherapy) will allow us to further improve outcomes for the vast majority of patients whose tumors lack an actionable biomarker. Decades after platinum-based therapy established itself as the standard of care, these tumor-specific therapies finally offer our patients a more efficacious, durable, and less toxic approach to care for their dire disease.
Acknowledgments
Funding: This work was funded in part through an American Cancer Society grant RSG 11-186 (to Daniel B. Costa) and a National Cancer Institute grant CA090578 (to Daniel B. Costa).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shaohua Cui (Department of Pulmonary Medicine, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.02.28). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv Nutr 2016;7:418-9. [Crossref] [PubMed]
- NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol 2008;26:4617-25. [Crossref] [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [Crossref] [PubMed]
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [Crossref] [PubMed]
- Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2013;31:2895-902. [Crossref] [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Crinò L, Ahn MJ, De Marinis F, et al. Multicenter Phase II Study of Whole-Body and Intracranial Activity With Ceritinib in Patients With ALK-Rearranged Non-Small-Cell Lung Cancer Previously Treated With Chemotherapy and Crizotinib: Results From ASCEND-2. J Clin Oncol 2016;34:2866-73. [Crossref] [PubMed]
- Ou SH, Ahn JS, De Petris L, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016;34:661-8. [Crossref] [PubMed]
- Shaw AT, Solomon BJ. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2015;372:683-4. [Crossref] [PubMed]
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol 2013;8:823-59. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Kerr KM, Tsao MS, Nicholson AG, et al. Programmed Death-Ligand 1 Immunohistochemistry in Lung Cancer: In what state is this art? J Thorac Oncol 2015;10:985-9. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Socinski M, Creelan B, Horn L, et al. NSCLC, metastaticCheckMate 026: A phase 3 trial of nivolumab vs investigator's choice (IC) of platinum-based doublet chemotherapy (PT-DC) as first-line therapy for stage iv/recurrent programmed death ligand 1 (PD-L1)−positive NSCLC. Ann Oncol 2016;27:LBA7. _PR. [Crossref]
- Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol 2017;12:208-22. [Crossref] [PubMed]
- Rangachari D, VanderLaan PA, Shea M, et al. Correlation between classic driver oncogene mutations in EGFR, ALK, or ROS1 and 22C3-PD-L1 ≥50% expression in lung adenocarcinoma. J Thorac Oncol 2017; [Epub ahead of print]. [Crossref] [PubMed]


