
Serum tumor markers predict cancer-related venous thromboembolism in gastric cancer
Introduction
Nowadays, gastric cancer, which is the fourth most common cancer and the second lethal cancer in the world, has become a critical healthy issue. Global cancer statistics reported 951,600 cases and 723,100 deaths in 2012 (1). Gastric cancer has the highest incidence in Eastern Asia area. It was estimated that 679,100 new cases arise and 498,000 patients died from gastric cancer in China in 2015. It also ranked the second lethal cancer in China (2). Even though undergoing a surgery, few patients could have a long-term survival after that. At the same time, chemotherapy is not radical cure for cancer. On the other hand, targeted therapy may play a significant part in the treatment of gastric cancer (3).
Metastasis is a primary cause of gastric cancer mortality (4). It proceeds through multiple steps and restrictive bottlenecks. Hematogenous metastasis is one of the most important ways. Cancer-related venous thromboembolism (CVT) is thought to be a critical step of hematogenous metastasis (5). Pathologically, vascular invasion can be defined as the visualization of CVT. According to the researches of hepatocellular carcinoma, vascular invasion has served as a relative contraindication for surgical resection. For gastric cancer, many studies regarded CVT only as a part of the pathological features or a factor associated with poor prognosis (6-8). Some researchers also thought CVT showed a statistically significant correlation with circulating tumor cell (CTC)-positivity, and strong predictors of poor survival in this disease (6,9).
Serum tumor marker is one factor associated with prognosis. Tumor marker was first proposed at Human Immunity and Tumor Immune Diagnosis Meeting in 1978. So far, the detection method of classic serum tumor markers has become a simple and low cost for detecting tumor and monitoring tumor progression. Clinically, carcinoembryonic antigen (CEA), cancer antigen (CA)19-9, CA242, CA72-4, and CA125 have been widely used for gastric cancer diagnosis (1,10-12).
CVT is one of high risk factors for poor prognosis of tumor. We hope to find a simple method to diagnose and predict CVT. CEA can be applied in the diagnosis and evaluation of tumor recurrence. In addition, it has been proved to have adhesive characteristic, suggesting that CEA expression on epithelial cells may directly influence tumor development by CEA-CEA bridges between tumor cells or tumor-stromal cells (13). Tumor markers of saccharide protein family, such as CA19-9, CA242, CA72-4, CA125, whether there is a relationship between their increase and CVT growth is still unclear. So we took relevant statistical analysis about preoperative tumor markers for gastric cancer and pathological factors of CVT.
Methods
Patients
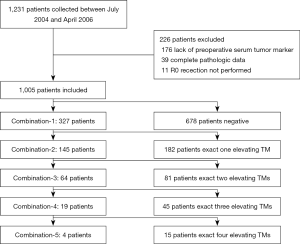
We retrospectively collected 1,231 patients with gastric cancer who underwent curative resection from July 2004 to April 2016 at the Department of General Surgery, Drum Tower Hospital Affiliated to Medical School of Nanjing University. Clinical and histopathological data, including age, gender, histology, stage, and histological grade were collected. A total of 1,005 patients were finally enrolled in this study, while 226 were excluded, among which 176 patients did not test the preoperative level of serum tumor marker, 39 patients did not have complete pathologic data, 11 patients who had R1 or R2 resection (Figure 1).
Detection of CVT
The status of CVT was detected by pathologists. It was confirmed when: (I) under the microscope, tumor invaded the vessel wall, intruded into the vessel lumen, and with a layer of vascular endothelium on the surface of the tumor thrombus; (II) by immunohistochemistry, CD34, a vascular endothelium marker, was positive; (III) if the blood vessel was a large one, elastic stain should also be carried out to look for the evidence of elastic fiber’s damage.
Measurement of serum tumor markers
Blood samples of enrolled patients were obtained by venipuncture within 1 week before curative surgery. CA19-9 and CA72-4 levels were investigated using electrochemiluminescence immunoassay (ECLIA), while others were investigated using chemiluminescence immunoassay (CLIA). We defined cut-off values to be 5 ng/mL for CEA, 39 U/mL for CA19-9, 15 U/mL for CA242, 6.9 U/mL for CA72-4, 30.2 U/mL for CA125. A result was considered as positive when the serum marker level was higher than the corresponding cut-off value. These assays were done at the Department of Clinical Laboratory, Drum Tower Hospital Affiliated to Medical School of Nanjing University. We also defined some subgroups according to the number of elevating tumor markers for each patient. Combination-1 subgroup included patients with at least one elevating tumor marker. Combination-2 subgroup included patients with at least two elevating tumor markers. Combination-3 subgroup included patients with at least three elevating tumor markers. Combination-4 subgroup included patients with at least four elevating tumor markers. Combination-5 subgroup included patients with five elevating tumor markers.
Statistical analyses
To analyze baseline characteristics, the categorical variables were compared with Pearson’s Chi-square test; the ranked variables were compared using a Wilcoxon test. The data for each tumor markers were express as mean ± SD for every subgroup. Measurement data between groups were compared with t-test. The odds ratios (ORs) and 95% confidence intervals (CIs) were calculated via both univariate and multivariate logistic regression models. The sensitivity (SEN), specificity (SPE), positive predictive values (PPV) and negative predictive values (NPV) of five biomarkers and the combination group were calculated, respectively.
Two-sided P values of less than 0.05 were considered as significant. SPSS 17.0 software was used for statistical analyses.
Results
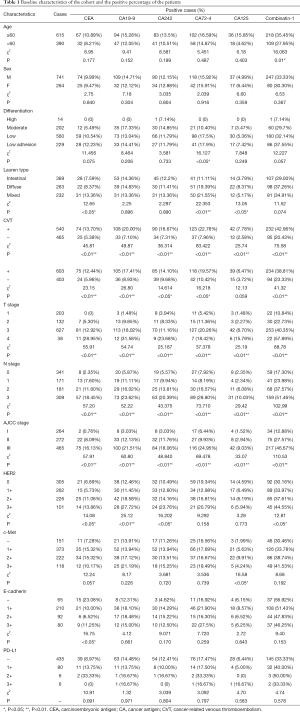
The study tested the preoperative level of serum tumor marker and collected postoperative pathologic data of 1,005 patients. The patients contained 741 male and 264 female with the median age of 61.5 years. CVT was found in 540 (53.73%) patients (Table 1).
Full table
From analyzing the relationship between preoperative serum tumor markers and main postoperatively pathological parameters of gastric cancer, it showed that serum levels of CEA and CA72-4 of patients who were suffering from poorly differentiated carcinoma and low adhesive cancer were significantly higher than those who suffered from high or medium differentiated carcinoma. The similar results were also shown in combined group. Grouped by Lauren type of gastric cancer, it displayed that the serum levels of CEA, CA72-4 and CA125 of mixed types and diffused types were higher than those of intestinal types. The increase of each cancer marker is remarkably related with both positive intravascular cancer emboli and nerve infiltration. Meanwhile, the increase of serum markers is correlated with higher T-stage, N-stage and pathological AJCC stage. It has also been confirmed the relationship between preoperative serum markers and some postoperative pathological immunohistochemistry results. For example, it can be referred that HER2 status was strongly linked with some tumor markers, such as CEA, CA19-9, and CA242. On the other hand, the status of c-Met, E-cadherin as well as HER2 were not so closely related with preoperative serum tumor markers. Furthermore, we compared the mean of every serum tumor marker of positive CVT patients with those of negative ones, the results showed that the means of positive ones were higher than negative ones on every marker (Table 2). As Table 2 illustrated that the gaps of CEA, CA19-9 and CA242 were obvious.

Full table
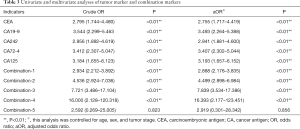
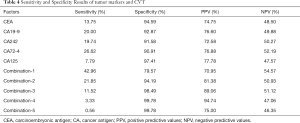
Both univariate and multivariate logistic regression models had been built to analyze the effects of increasing serum tumor markers on positive CVT (Table 3). Table 3 showed the rise of serum tumor markers could be regarded as a negative factor for positive CVT. The more serum tumor markers come out, the higher the risk of positive CVT can be. The incidence of positive CVT would be 2.9 times that of a normal patient with any one of the tumor markers increases. With the increase of any 4 of the tumor markers can make the risk rise 16.4 times. The sensibility and specificity for positive CVT have been calculated by analyzing tumor markers separately as well as in combination (Table 4). It can be projected from Chart 4 that the specificity of tumor markers was as higher as the more abnormal tumor markers there were. The specificity can be up to 95.7% when at least 4 tumor markers were used to predict. On the other hand, when it was predicted by tumor markers separately, the result of sensibility for positive CVT was not ideal which specificity was 43.0% at most.
Full table
Full table
Discussion
The results of this study showed that there were at least one tumor marker elevation in 42.96% of the postoperative patients with CVT.
Most gastric cancers are gastric adenocarcinomas (3). Our study focused on the relationship between preoperative serum tumor markers of patients with gastric adenocarcinomas, such as CEA, CA19-9, CA242, CA72-4, CA125 with common clinical or pathological parameters. And we found that there was a correlation between the five tumor markers and postoperative CVT. Any one of the five tumor markers was used to predict the positivity of CVT had high specificity, and combination of multiple tumor markers to predict the positivity of CVT had an improved sensitivity.
The clinical symptoms of gastric cancer are atypical, many patients may miss the opportunity of curative surgical treatment at the first diagnosis (11,12,14). The diagnosis of gastric cancer relies on histomorphological and primarily the domain of surgical pathologists. Even though the current imaging examinations including computed tomography (CT), magnetic resonance imaging (MRI), endoscopic ultrasonography (EUS) can be used to assess whether the partial patient should undergo radical surgery or not, these methods are difficult to assess whether the existence of CVT. In recent years, there are more and more serum biomarkers discovered for gastric cancer patients (15-19). Detection of serum tumor markers are an inexpensive, convenient method that patients may afford, however, the sensitivity and specificity of the detection are not satisfactory for clinical use. Their use in gastric cancer can be acknowledged in: (I) monitoring the effectiveness of cytostatics, while the gold standard remains to be radiological assessment; (II) the follow-up period, however their role is currently under debate because an early detection of relapse does not improve survival rates (10). Many studies have shown that for patients with gastric cancer, the positivity of CEA, CA19-9, and CA72-4 are associated with many clinicopathological factors, including tumor infiltration depth, lymph node involvement, peritoneal metastases, and cancer stage (7,8,12). Despite the positive rates of the three serum tumor markers are similar, some research have shown that CA72-4 is the best among the three (20,21). In our study, serum CA72-4 has no advantage relativity with tumor differentiation, or Lauren types. The possible reason may be that our study is a retrospective study that our patients were only received curative surgery, most of which were not stage IV disease. Besides clinicopathological factors, serum tumor marker always have relationship with cancer recurrence and prognosis in the patients with gastric cancer (22,23). In terms of recurrence and prognosis of gastric cancer, the sensitivity and specificity of serum tumor markers are much higher than for the diagnosis. Serum tumor markers can also be used as monitor markers during treatment of gastric cancer, CEA and CA19-9 are the common markers which are used as monitoring indicators (11,24). In addition we also found that the relationship between clinicopathological parameters with any one of the five tumor markers separately and the combination of some serum tumor markers may be more useful in clinical work. Some studies discussed that combination of two or three tumor markers, and the results showed that the combination of tumor markers to assess the status of the patients may have a better effect (25-27). Therefore, in our study, we investigated the statistic relationship between preoperative serum level of the five tumor markers and clinicopathological characteristics of gastric cancer. Furthermore, for CVT we not only investigated each one of the five tumor markers, but also the combination. The result showed that combination-1 group, which including patients with at least one elevating tumor marker, has the highest sensitivity to predict postoperative CVT.
Why are serum tumor markers associated with CVT? We concerned about this because the tumor has a “homotypic targeting”. Coated with the source cancer cell membrane specifically derived from the homologous tumors, the nanoparticles are identified with the self-recognition internalization by the source cancer cell lines in vitro and the highly tumor-selective targeting “homing” to the homologous tumor in vivo (28). Metastasis is associated with cancer-related death, while blood metastasis is an important part of tumor metastasis. In situ tumor infiltrate vessel by a series of biological behavior changes and form CVT (5). At present, only a few researches focus on the effect of CEA on the formation of the CVT. CEA is a member of the immunoglobulin superfamily. It’s a highly glycosylated cell surface glycoprotein, of molecular weight 180,000 (protein, 72,800), and is expressed at greatly increased the levels in nearly all human colon carcinomas (29). Some studies on CEA also found it as a kind of adhesion molecules, which can mediate tumor adhesion in vivo (13,30). When vascular endothelial cells contact with tumor cells expressing CEA, vascular endothelial cells will also express CEA, then the CEA expression cell could adhere another cell with the similar CEA expression and form cell-cell adhesion, the adhesion could finally form CVT (13). The tumor cell which has already entered the blood vessel is now considered to belong to the CTCs (5,9). Once the formation of tumor thrombus, its invasion ability is much more than a single CTC, and the risk of distant metastasis will significantly increase. Therefore, CVT is also considered to be one of the poor prognosis factors of tumor. Recent studies about CVT concentrated in liver cancer showed the diagnosis and treatment of portal vein tumor thrombus and microscopic tumor thrombus. Some studies have indicated that the risk factors for early postoperative recurrence of hepatocellular carcinoma are the microvascular invasion. The 1-year recurrence rate of patients with radical resection is from 22% to 75% (31-34). In gastric cancer, the presence of CVT is also related to the prognosis, but the researches are few. The results of our study show that 540 (53.73%) were with positive with CVT among 1,005 patients. We hope that we could predict the status of postoperative CVT from preoperative serum tumor markers. For those patients with a high risk of positive CVT, more active treatment should be arranged, such as preoperative neoadjuvant chemotherapy to reduce the positive risk of CVT and prolong the postoperative survival after radical surgery. Furthermore, it will improve the treatment effect in those patients who would take a CTCs detection or carry drugs through the individual tumor cells. And maybe we also can use serum tumor marker to find a strategy for cancer immunotherapy (35).
At present, the clinical research of tumor markers is focused on the prediction of tumor screening and recurrence. But there is no report about the relationship of markers of tumor and CVT, and its mechanism needs further investigation and we may find a new method to prevent of CVT formation and tumor metastasis.
Acknowledgments
Funding: This study was funded by the Funds for Major International (Regional) Joint Research Project of the Natural Science Foundation of China (grant number 81220108023), Natural Science Foundation of China (grant number 81370064) and the Natural Science Foundation for Distinguished Yong Scholars of China (grant number 81401969).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.03.31). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of Nanjing Drum Tower Hospital of Nanjing University Medical School (NO. 2014-019-02). Written informed consent was obtained from the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet 2016;388:2654-64. [Crossref] [PubMed]
- Li M, Hong G, Cheng J, et al. Identifying Reproducible Molecular Biomarkers for Gastric Cancer Metastasis with the Aid of Recurrence Information. Sci Rep 2016;6:24869. [Crossref] [PubMed]
- Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 2009;9:274-84. [Crossref] [PubMed]
- Kooby DA, Suriawinata A, Klimstra DS, et al. Biologic predictors of survival in node-negative gastric cancer. Ann Surg 2003;237:828-35; discussion 835-7. [Crossref] [PubMed]
- Araki I, Hosoda K, Yamashita K, et al. Prognostic impact of venous invasion in stage IB node-negative gastric cancer. Gastric Cancer 2015;18:297-305. [Crossref] [PubMed]
- Du CY, Chen JG, Zhou Y, et al. Impact of lymphatic and/or blood vessel invasion in stage II gastric cancer. World J Gastroenterol 2012;18:3610-6. [Crossref] [PubMed]
- Maltoni R, Fici P, Amadori D, et al. Circulating tumor cells in early breast cancer: A connection with vascular invasion. Cancer Lett 2015;367:43-8. [Crossref] [PubMed]
- Waddell T, Verheij M, Allum W, et al. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol 2014;40:584-91. [Crossref] [PubMed]
- Dilege E, Mihmanli M, Demir U, et al. Prognostic value of preoperative CEA and CA 19-9 levels in resectable gastric cancer. Hepatogastroenterology 2010;57:674-7. [PubMed]
- Ucar E, Semerci E, Ustun H, et al. Prognostic value of preoperative CEA, CA 19-9, CA 72-4, and AFP levels in gastric cancer. Adv Ther 2008;25:1075-84. [Crossref] [PubMed]
- Benchimol S, Fuks A, Jothy S, et al. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell 1989;57:327-34. [Crossref] [PubMed]
- Reiter W, Stieber P, Reuter C, et al. Prognostic value of preoperative serum levels of CEA, CA 19-9 and CA 72-4 in gastric carcinoma. Anticancer Res 1997;17:2903-6. [PubMed]
- Zheng ZX, Sun Y, Bu ZD, et al. Intestinal stem cell marker LGR5 expression during gastric carcinogenesis. World J Gastroenterol 2013;19:8714-21. [Crossref] [PubMed]
- Wu Y, Wang KY, Li Z, et al. Y-box binding protein 1 expression in gastric cancer subtypes and association with cancer neovasculature. Clin Transl Oncol 2015;17:152-9. [Crossref] [PubMed]
- Cai H, Yuan Y, Hao YF, et al. Plasma microRNAs serve as novel potential biomarkers for early detection of gastric cancer. Med Oncol 2013;30:452. [Crossref] [PubMed]
- Chan SH, Wu CW, Li AF, et al. miR-21 microRNA expression in human gastric carcinomas and its clinical association. Anticancer Res 2008;28:907-11. [PubMed]
- Yamanaka S, Olaru AV, An F, et al. MicroRNA-21 inhibits Serpini1, a gene with novel tumour suppressive effects in gastric cancer. Dig Liver Dis 2012;44:589-96. [Crossref] [PubMed]
- Shimada H, Noie T, Ohashi M, et al. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer 2014;17:26-33. [Crossref] [PubMed]
- Chen XZ, Zhang WK, Yang K, et al. Correlation between serum CA724 and gastric cancer: multiple analyses based on Chinese population. Mol Biol Rep 2012;39:9031-9. [Crossref] [PubMed]
- Wang Q, Yang Y, Zhang YP, et al. Prognostic value of carbohydrate tumor markers and inflammation-based markers in metastatic or recurrent gastric cancer. Med Oncol 2014;31:289. [Crossref] [PubMed]
- Wang W, Chen XL, Zhao SY, et al. Prognostic significance of preoperative serum CA125, CA19-9 and CEA in gastric carcinoma. Oncotarget 2016;7:35423-36. [PubMed]
- Kim DH, Oh SJ, Oh CA, et al. The relationships between perioperative CEA, CA 19-9, and CA 72-4 and recurrence in gastric cancer patients after curative radical gastrectomy. J Surg Oncol 2011;104:585-91. [Crossref] [PubMed]
- Stiksma J, Grootendorst DC, van der Linden PW. CA 19-9 as a marker in addition to CEA to monitor colorectal cancer. Clin Colorectal Cancer 2014;13:239-44. [Crossref] [PubMed]
- Yang AP, Liu J, Lei HY, et al. CA72-4 combined with CEA, CA125 and CAl9-9 improves the sensitivity for the early diagnosis of gastric cancer. Clin Chim Acta 2014;437:183-6. [Crossref] [PubMed]
- Nagler R, Bahar G, Shpitzer T, et al. Concomitant analysis of salivary tumor markers--a new diagnostic tool for oral cancer. Clin Cancer Res 2006;12:3979-84. [Crossref] [PubMed]
- Zhu JY, Zheng DW, Zhang MK, et al. Preferential Cancer Cell Self-Recognition and Tumor Self-Targeting by Coating Nanoparticles with Homotypic Cancer Cell Membranes. Nano Lett 2016;16:5895-901. [Crossref] [PubMed]
- Shuster J, Thomson DM, Fuks A, et al. Immunologic approaches to diagnosis of malignancy. Prog Exp Tumor Res 1980;25:89-139. [PubMed]
- Screaton RA, Penn LZ, Stanners CP. Carcinoembryonic antigen, a human tumor marker, cooperates with Myc and Bcl-2 in cellular transformation. J Cell Biol 1997;137:939-52. [Crossref] [PubMed]
- Tanaka S, Mogushi K, Yasen M, et al. Surgical contribution to recurrence-free survival in patients with macrovascular-invasion-negative hepatocellular carcinoma. J Am Coll Surg 2009;208:368-74. [Crossref] [PubMed]
- Kim H, Park MS, Park YN, et al. Preoperative radiologic and postoperative pathologic risk factors for early intra-hepatic recurrence in hepatocellular carcinoma patients who underwent curative resection. Yonsei Med J 2009;50:789-95. [Crossref] [PubMed]
- Wang GB, Zhou XY, Wang XQ. Relationship between serum heparanase and microscopic venous invasion in patients with hepatocellular carcinoma. Am J Clin Pathol 2010;134:242-8. [Crossref] [PubMed]
- You Z, Chen LP, Ye H. Predictors of microvascular invasion in patients with solitary small hepatitis B related hepatocellular carcinoma. Pak J Med Sci 2014;30:331-4. [Crossref] [PubMed]
- Xiao H, Woods EC, Vukojicic P, et al. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc Natl Acad Sci U S A 2016;113:10304-9. [Crossref] [PubMed]


