
Transient receptor potential, Melastatin-2 (TRPM2) blockade: perspectives on potential novel clinical utility in cancer
Introduction
Cancers that originate in the tissues of the head and neck affect 650,000 new patients every year with 350,000 cases resulting in fatalities annually (1). The majority of these “head and neck” cancers begin in squamous cells (2), which line the layer of the epidermis superior to the basal cell layer. Amongst head and neck cancers, the most common type of oral cancer is squamous cell carcinoma (SCC). Treatment modalities for these patients include apoptosis induction, inhibition of cancer invasion, and functioning to reduce the complex processes of cancer metastasis (1). Transient receptor potential melastatin-2 (TRPM2) is one ion channel that can be altered to increase apoptosis in cancer cells. TRPM2, a member of the transient receptor potential (TRP) melastatin subfamily of cellular sensors, is presented in a recent article in Scientific Reports due to its affiliation with inhibition of salivary gland function and expression associated with SCC.
Results from this study further uncovered the expression, function, and mechanism of TRPM2 in SCC. Through the use of immunohistochemistry, Zhao et al. observed that the expression of TRPM2 was more prevalent among tongue carcinoma samples (22 out of 23 samples) than papilloma of tongue specimens (6/12) and normal tongue tissue samples (0/9) (3). It was also observed that peroxide concentrations were directly proportional to levels of cell apoptosis in SCC—linked to the fact that TRPM2 channels are activated by oxidative stress. However, inhibiting TRPM2 by introduction of anti-TRPM2 short hairpin RNA (shRNATRPM2) was also shown to increase apoptosis in SCC cells. Ion channels, such as TRPM2, play a vital role in mandatory cell functions, but this also suggests that certain ion channels that are unique to cancer cells can be manipulated to induce apoptosis in these cells. Because Zhao et al. found that TRPM2 was overexpressed in SCC, TRPM2 certainly appears to fit the criteria as one such ion channel. Through showing that TRPM2 can be activated by oxidative stress, the authors, among other studies, discussed how this channel can be used in mechanisms characterized by oxidative environments in many diseases. The differences in transcripts and location of TRPM2 channels were also shown to have an impact on the function and pathways of TRPM2. Taken together, these observations indicate that further research on the roles of TRPM2 channels in cancer and the evaluation of the pharmacological inhibition of TRPM2 in these cancers may possibly lead to improved and selective treatment regimens in the future.
TRPM2 roles in oral cancer
To initially validate their studies, Zhao et al. verified that the TRPM2 channels were in fact expressed in the cells being studied. To accomplish this, they employed the use of immunohistochemistry, real-time or semiquantitative polymerase chain reaction (PCR), and Western blot analyses on malignant and non-malignant tongue specimens, as well as human tongue SCC lines. The results not only revealed the presence of TRPM2, but also statistically significant increases in TRPM2 mRNA expression and protein levels in malignant tongue specimens as compared to non-malignant samples. Their results were congruent with their initial hypothesis that TRPM2 channels are present in greater quantity in cancer cells—a characteristic that possibly has proliferative and migratory effects on cancer cells.
To further validate this overexpression, SCC cells were shown to exhibit greater levels of TRPM2 ion currents when induced by adenosine diphosphoribose (ADP-ribose), a well-known activator of TRPM2 ion channels (4). In addition, Zhao et al. confirmed that the TRPM2 channel was functional in these cancer cells by investigating the known role of the channel in mediating oxidative stress-induced cell death. By subjecting the cancerous cells to H2O2 (a known activator of TRPM2 channels) (5), the authors confirmed normal functioning of the channels due to the downstream observations of increased levels of activated caspase-3 and -9, and decreased levels of Bcl-2, p53, and p21. Consequently, H2O2-induced TRPM2 channel activation led to the induction of apoptosis in both the cancerous and normal cell lines. These results thus verified that TRPM2 was indeed functional in cancerous cell lines.
TRPM2 roles in other cancers
Collectively, TRPM channels have been identified as therapeutic targets or otherwise playing important roles in several types of cancer. For instance, TRPM7 was identified to have potential roles in gastric cancer (6), pancreatic ductal carcinoma (7), and nasopharyngeal carcinoma (8), while TRPM8 expression has been associated with prostate (9), pancreatic (10), and breast cancer (11). However, with the inclusion of the recent report by Zhao et al., TRPM2 channels alone have now been identified as playing essential roles in six types of cancer (Table 1). These potential roles include: the facilitation of survival, proliferation, and genomic stability in breast adenocarcinoma cells (12,17); the promotion of survival and metastasis in head and neck cancer (3); the facilitation of NF-κB function in acute myeloid leukemia (AML) (13); the facilitation of proliferation and/or survival in melanoma and prostate cancer (14,16); and the promotion of survival and mitochondrial function in neuroblastoma (15). However, it is worth noting that the abolishment of TRPM2 function in AML failed to increase sensitivity to chemotherapeutic agents (discussed later). Taken together, these studies indicate that TRPM2 channels perform pivotal roles in many different types of cancers. Thus, current and previous research have identified TRPM2 channels as novel targets for the treatment of a multitude of human cancers.

Full table
Cellular localization of TRPM2
The subcellular localization of TRPM2 appears to differ in cancerous versus non-cancerous cells. The recent report by Zhao et al. demonstrates a nuclear localization of TRPM2 in SCC9 cells via immunofluorescence microscopy and human tongue carcinoma samples via immunoblot analysis after nuclear extractions. However, a cellular membrane localization has been noted in normal human tongue samples. Previously, TRPM2 was shown to be primarily localized to the plasma membrane in noncancerous cells, with lesser amounts in the cytoplasm (18). Also, TRPM2 splice variants were reported (19,20), but all were observed in the cytoplasm or endoplasmic reticulum. An alternative splicing product was observed in mice that has a peri-nuclear localization (21), but no such TRPM2 variant was observed in human cells. Thus, current studies support the concept that TRPM2 is localized to the cytoplasm or plasma membrane in non-cancerous cells, presumably to function as an ion channel.
However, recent literature reports indicate that TRPM2 is present in the nuclei of several different types of cancer cells (Table 2), presumably to function in a new, yet unknown physiologic activity. Zeng et al. first reported a localization of TRPM2 in human prostate cancer cell nuclei, but not in non-cancerous human prostate cell nuclei (16). We then reported a nuclear localization of full-length TRPM2 in two lines of human breast adenocarcinoma cells, while its localization in non-cancerous human breast cells exhibited a cytoplasmic/plasma membrane localization (12). We subsequently demonstrated similar results in four human metastatic melanoma cell lines, where TRPM2 was shown to reside in the nucleus, but it displayed a cytoplasmic/plasma membrane localization in non-cancerous human skin cells (14). However, in neuroblastoma, Bao et al. found that the localization of TRPM2 was in fact cytoplasmic, where it was reported to promote survival and mitochondrial function (15). Taken together, it appears that TRPM2 has an important nuclear role in several cancers (breast, head and neck, melanoma, and prostate), while in others, such as neuroblastoma, its function may be exclusively required in the cytoplasm or at the plasma membrane.
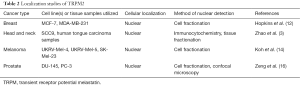
Full table
It is possible that the difference in localization in cancerous versus non-cancerous cells is responsible for the varying function of TRPM2 in mediating either cell death in non-cancerous cells or proliferation, survival, or migration in cancerous cells. The mechanism by which TRPM2 produces the latter set of consequences, which are essential for cancer cells to propagate or metastasize, is unknown. However, the discovery of this mechanism is potentially the most intriguing aspect of TRPM2 function in cancer cells, as the finding would not only be novel, but may provide insight into an additional pathway that cancer cells exploit to initiate or maintain the cancer phenotype. For example, the data implies, as suggested by Zhao et al., that the function and role of TRPM2 depends on its location. When localized to the plasma membrane, TRPM2 may behave in a protective manner by inhibiting malignant cell types from proliferating in initial stages of disease onset (i.e., promoting apoptosis when activated). On the contrary, when TRPM2 channels are located in greater quantity in the nucleus, they may contribute to significant advancement in disease progression. Thus, the intranuclear mechanism of events that would permit this could be the most groundbreaking discovery.
Moving forward, important studies will need to elucidate the physiologic function(s) of TRPM2 in the nucleus of cancer cells. Is TRPM2 an ion channel at the nuclear membrane? Although plasma membrane TRPM2 channels gate cations—including Ca+ ions—into the cell from the extracellular milieu, it is unlikely that they act in a similar manner at the nuclear membrane, as the pore structure of the nuclear membrane would allow the free passage of ions into the nucleus. However, this possibility cannot be completely dismissed. Another possibility is the ability of TRPM2 to facilitate or participate in DNA repair. We previously provided evidence for this role when we demonstrated that inhibition or RNAi silencing of TRPM2 caused extensive levels of DNA damage, as measured by the Comet assay (12). An additional possibility is the ability of TRPM2 to act as a transcription factor in order to activate genes required for propagation, proliferation, or migration. Although TRPM2 was shown to indirectly modulate transcription in neuroblastoma and leukemia (14,15), no studies to date have shown the ability of TRPM2 to directly regulate DNA transcription. Finally, other possibilities include a role for TRPM2 in nuclear import/export, the ability to act as a molecular chaperone, or a structural role as a chromatin-binding protein (although there is currently no evidence that supports these roles).
Inhibition of TRPM2 function
TRPM2 is a non-specific cation channel that is well-known for the gating of Ca+ ions into the cell. The gating of Ca+ in response to oxidative stress leads to the Ca+-mediated facilitation of cell death pathways (22). TRPM2 has thus been shown to have key roles in exacerbating inflammation (23) and cell death after oxidative stress. Pharmacologic inhibition of TRPM2 is currently being studied for its ability to provide protective effects in response to acute pathological conditions (24), neurodegenerative disease (5), and diabetes (18). While TRPM2 inhibition is currently studied for its protective effects in noncancerous cells, the recent report by Zhao et al. and other independent research groups demonstrate an opposite effect in cancer, where TRPM2 inhibition led to decreased proliferation and migration, and increased cancer cell death after chemotherapy. Because of the implications of TRPM2 on disease progression and cell survival, blockade of TRPM2 channels may provide a significant impact on cancer treatment research.
In this particular study, Zhao et al. inactivated the channels via two different means: pharmacologic blockade and shRNA. The former was achieved via administering known inhibitors of TRPM2. More significantly, the authors utilized shRNATRPM2 to promote knockdown of TRPM2. Their results showed a statistically significant decrease in TRPM2 levels, an increase in apoptotic cells, and a decrease in migration as compared to control. All of these results correlate directly to the channel’s role in disease progression; in blocking these channels, malignant cells are less prone to advanced proliferation and metastasis. Furthermore, the inhibition of these channels increases the effectiveness of chemotherapy regimens.
As previously noted, the absence of TRPM2 in AML produced no significant increases in the death of leukemia cells after various chemotherapeutic treatments (14). Although TRPM2 was shown to be overexpressed in these leukemia cells, studies analyzing the cellular localization of TRPM2 were not presented. Consequently, it is possible that TRPM2 does not exhibit a nuclear localization in leukemia cells. Thus, the failure to observe the desired chemotherapeutic effects due to the absence of TRPM2 in leukemia may reflect that TRPM2 does not perform an essential nuclear function in these cells.
Clinical relevance
Taken together, the results of the study by Zhao et al. and the discoveries of other laboratories hold significant potential for improving the treatment of a variety of cancers. The TRPM family, especially the TRPM2 subfamily member, possess certain characteristics that make them ideal targets for cancer therapy. Many conventional chemotherapeutic agents are non-specific, as they target both cancer cells and rapidly dividing normal cells (such as hair follicles and oropharyngeal epithelial cells), causing a milieu of unpleasant side effects. Additionally, if taken at elevated doses, chemotherapy can be extremely debilitating and potentially fatal. Targeting TRPM2 potentially allows for improved specificity, increasing the toxic killing power of conventional chemotherapeutics.
TRPM2 provides this desired specificity through a variety of mechanisms. Namely, TRPM2 is overexpressed in SCC9 and SCC25 cells (as well as in other cancer cell types as discussed previously) as compared to control, increasing cell cycle effects upon TRPM2 blockade (3). Additionally, TRPM2 demonstrates both a unique nuclear localization and novel function in cancerous cell lines as compared to healthy controls. In cancerous cells, TRPM2 serves protective and proliferative roles, promoting growth and migration and potentially the cancer phenotype. Conversely, TRPM2 promotes apoptotic cell death upon activation by oxidative stress in healthy cells. Thus, blockade of TRPM2 in cancerous cells, either pharmacologically or via shRNATRPM2, promotes increased cell death while blocking oxidative stress-induced cell death in healthy cells. This function differential is key for the ability to selectively target cancerous cells for eradication.
Clinically, therapeutic blockade of TRPM2 channels holds significant promise. If effective TRPM2 inhibitors can be designed, regimens could be fashioned to utilize a TRPM2 inhibitor first, priming the cancer cells for chemotherapy by blocking these channels that promote survival, followed by conventional chemotherapy. As a result of the selective nature of TRPM2 inhibition, as explained by both its unique nuclear localization and function, blocking these channels will allow for direct cell-killing effects of cancer cells while minimizing harmful effects to healthy cells. In addition, TRPM2 blockade may reduce the minimum effective doses of various chemotherapeutic agents, thus minimizing the unwanted deleterious side effects of chemotherapy. In summary, the use of TRPM2 blockade, whether pharmacologically or via more permanent means, holds significant potential for improving the effectiveness of current chemotherapeutic regimens and for improving the lives of cancer patients.
Future studies
While the additive/synergistic potential of combining therapeutic TRPM2 blockade with chemotherapy is immensely promising, two significant milestones must be attained. The first, as was discussed in the previous section, is to determine the nuclear function of TRPM2 in cancer cells. The second is to determine the effects of TRPM2 inhibition in whole animal cancer models. While tested in a multitude of cultured cancer cell lines, TRPM2 inhibition has yet to be tested in vivo. Only in vitro applications have been investigated, with the exception of the study performed by Haladyna et al., in which an AML transplantation model on a TRPM2-null background was utilized. Again, although the desired results were not observed in this particular study, the possibilities for these results were previously discussed. Finally, another consideration concerning future therapeutic use of TRPM2 blockade is the exact method of achieving such blockade. While pharmacologic agents can be used to block TRPM2 (13,25), more specific and selective inhibitors would be required. Thus, important future studies would include the rational design and evaluation of novel TRPM2 pharmacologic inhibitors. Therefore, the completion of these future studies will help elucidate the full spectrum of the function and potential of targeting TRPM2 in various types of human cancers in the future.
Conclusions
In conclusion, the recent report by Zhao et al. provides further evidence of a novel role for the TRPM2 channel in several types of cancer. The importance of TRPM2 function in these cancers is highlighted by its overexpression in some of these cancer types and the ability of TRPM2 inhibition to prevent proliferation, survival, and migration of these cancer cells. To date, TRPM2 appears to have an important role in breast cancer, head and neck cancer, melanoma, and prostate cancer, where this novel role appears to originate in the nuclei of these respective cancer cells. As there is growing evidence that specific types of cancer contain specific vulnerabilities, the presence of nuclear TRPM2 may represent such a vulnerability. Therefore, the knowledge gained from investigating nuclear TRPM2 can potentially be exploited to improve the treatment of human cancers in the future.
Acknowledgments
Funding: This article and the preliminary research results reported were supported in part by the Bower, Bennet and Bennet Endowed Chair Research Award at Ohio Northern University.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Chengzhong Cai (National Center for Toxicological Research, Food and Drug Administration, USA; Shanghai 10th People’s Hospital, Tongji University, Shanghai, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.03.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Noguti J, De Moura CF, De Jesus GP, et al. Metastasis from oral cancer: an overview. Cancer Genomics Proteomics 2012;9:329-35. [PubMed]
- Argiris A, Karamouzis MV, Raben D, et al. Head and neck cancer. Lancet 2008;371:1695-709. [Crossref] [PubMed]
- Zhao LY, Xu WL, Xu ZQ, et al. The overexpressed functional transient receptor potential channel TRPM2 in oral squamous cell carcinoma. Sci Rep 2016;6:38471. [Crossref] [PubMed]
- Perraud AL, Fleig A, Dunn CA, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 2001;411:595-9. [Crossref] [PubMed]
- Fonfria E, Marshall IC, Boyfield I, et al. Amyloid beta-peptide(1-42) and hydrogen peroxide-induced toxicity are mediated by TRPM2 in rat primary striatal cultures. J Neurochem 2005;95:715-23. [Crossref] [PubMed]
- Kim BJ, Park EJ, Lee JH, et al. Suppression of transient receptor potential melastatin 7 channel induces cell death in gastric cancer. Cancer Sci 2008;99:2502-9. [Crossref] [PubMed]
- Rybarczyk P, Gautier M, Hague F, et al. Transient receptor potential melastatin-related 7 channel is overexpressed in human pancreatic ductal adenocarcinomas and regulates human pancreatic cancer cell migration. Int J Cancer 2012;131:E851-61. [Crossref] [PubMed]
- Qin Y, Liao ZW, Luo JY, et al. Functional characterization of TRPM7 in nasopharyngeal carcinoma and its knockdown effects on tumorigenesis. Tumour Biol 2016;37:9273-83. [Crossref] [PubMed]
- Tsavaler L, Shapero MH, Morkowski S, et al. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res 2001;61:3760-9. [PubMed]
- Yee NS, Zhou W, Lee M. Transient receptor potential channel TRPM8 is over-expressed and required for cellular proliferation in pancreatic adenocarcinoma. Cancer Lett 2010;297:49-55. [Crossref] [PubMed]
- Chodon D, Guilbert A, Dhennin-Duthille I, et al. Estrogen regulation of TRPM8 expression in breast cancer cells. BMC Cancer 2010;10:212. [Crossref] [PubMed]
- Hopkins MM, Feng X, Liu M, et al. Inhibition of the transient receptor potential melastatin-2 channel causes increased DNA damage and decreased proliferation in breast adenocarcinoma cells. Int J Oncol 2015;46:2267-76. [PubMed]
- Haladyna JN, Pastuer T, Riedel SS, et al. Transient potential receptor melastatin-2 (Trpm2) does not influence murine MLL-AF9-driven AML leukemogenesis or in vitro response to chemotherapy. Exp Hematol 2016;44:596-602.e3. [Crossref] [PubMed]
- Koh DW, Powell DP, Blake SD. Abstract 1269: Enhanced cytotoxicity in primary human metastatic melanoma cells via inhibition of the transient receptor potential melastatin-2 (TRPM2) channel. Cancer Research 2016;76:Abstract nr 1269.
- Bao L, Chen SJ, Conrad K, et al. Depletion of the Human Ion Channel TRPM2 in Neuroblastoma Demonstrates Its Key Role in Cell Survival through Modulation of Mitochondrial Reactive Oxygen Species and Bioenergetics. J Biol Chem 2016;291:24449-64. [Crossref] [PubMed]
- Zeng X, Sikka SC, Huang L, et al. Novel role for the transient receptor potential channel TRPM2 in prostate cancer cell proliferation. Prostate Cancer Prostatic Dis 2010;13:195-201. [Crossref] [PubMed]
- Koh DW, Powell DP, Blake SD, et al. Enhanced cytotoxicity in triple-negative and estrogen receptor positive breast adenocarcinoma cells due to inhibition of the transient receptor potential melastatin-2 channel. Oncol Rep 2015;34:1589-98. [PubMed]
- Lange I, Yamamoto S, Partida-Sanchez S, et al. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci Signal 2009;2:ra23. [Crossref] [PubMed]
- Kühn FJ, Kühn C, Naziroglu M, et al. Role of an N-terminal splice segment in the activation of the cation channel TRPM2 by ADP-ribose and hydrogen peroxide. Neurochem Res 2009;34:227-33. [Crossref] [PubMed]
- Uemura T, Kudoh J, Noda S, et al. Characterization of human and mouse TRPM2 genes: identification of a novel N-terminal truncated protein specifically expressed in human striatum. Biochem Biophys Res Commun 2005;328:1232-43. [Crossref] [PubMed]
- Hofmann T, Schaefer M, Schultz G, et al. Cloning, expression and subcellular localization of two novel splice variants of mouse transient receptor potential channel 2. Biochem J 2000;351:115-22. [Crossref] [PubMed]
- Hara Y, Wakamori M, Ishii M, et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 2002;9:163-73. [Crossref] [PubMed]
- Yamamoto S, Shimizu S, Kiyonaka S, et al. TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med 2008;14:738-47. [Crossref] [PubMed]
- Takahashi K, Sakamoto K, Kimura J. Hypoxic stress induces transient receptor potential melastatin 2 (TRPM2) channel expression in adult rat cardiac fibroblasts. J Pharmacol Sci 2012;118:186-97. [Crossref] [PubMed]
- Kraft R, Grimm C, Frenzel H, et al. Inhibition of TRPM2 cation channels by N-(p-amylcinnamoyl)anthranilic acid. Br J Pharmacol 2006;148:264-73. [Crossref] [PubMed]


