Effect of fasting therapy in chemotherapy-protection and tumor-suppression: a systematic review
Introduction
Cancer is the second leading cause of mortality worldwide and suspected to be the foremost killer in the coming decades by the World Health Organization (1). Cancer treatments including surgery, chemotherapy and radiotherapy has achieved considerable therapeutic efficacy, but damage to the normal tissue and the subsequent side-effects is inevitable, and the further improvement of prognosis is still challenging. Accordingly, besides the conventional therapy modalities, it is important to identify other assistant treatment methods to further enhance the therapeutic efficacy, reduce side-effects and improve prognosis. A growing number of recent studies in cancer treatments have suggested that factors in the categories of naturopathic medicine such as physical activity (2-4), psychological emotion (5), and healthy diet (6,7) have a profound effect on the initiation and treatment outcomes of cancer.
Fasting therapy is a naturopathic treatment method that has been used as a valid therapeutic modality for acute and chronic diseases in traditional medicine worldwide. In a most recent study, Wang et al. confirmed that fasting was protective while nutritional supplementation was detrimental in mice with bacterial sepsis (8). As in cancer-bearing models, fasting therapy was reported to be a reproducible and efficient intervention strategy in protecting mammals against tumor and prolonging overall survival (9,10). Besides, the chemotherapy-protection effects of fasting therapy in reducing chemotherapy side-effects and related death were also suggested in experiments (11,12).
However, controversy still existed in this field with inconsistent results reported. Besides, studies focusing on this issue have not been systematically reviewed and summarized. Therefore, we performed the current systematic review to combine published studies and to comprehensively evaluate the potential effects of fasting therapy in chemotherapy-protection and tumor-suppression.
Methods
Comprehensive literature searches were conducted in PubMed, Embase, Web of Science and Cochrane Library with no restriction to language and date of publication. The last search was conducted on Jul 3, 2016. The search terms were as follows: (“fasting”(mesh) or “starvation”(mesh)) and (“tumor” or “neoplasm” or “cancer” or “sarcoma” or “malignancy” or “carcinoma”) and (“prognosis” or “prognostic” or “predictive” or “survival” or “outcome” or “mortality” or “growth” or “progression” or “ proliferation” or “size” or “weight” or “volume” or “metastasis” or “chemotherapy” or “side effects” or “vomiting” or “diarrhea” or “immunosuppression”). In addition, reference lists of identified studies were traced by Google Scholar for potential studies.
Assessment of eligibility was undertaken independently by two authors. Studies were eligible for inclusion if the following criteria were met: (I) peer-reviewed research articles based on animals or humans; (II) presented the correlations of fasting therapy with chemotherapy side-effects (e.g., weight loss, vomiting, nausea, diarrhea, fatigue, cardiac function, bone marrow suppression, and chemotherapy-induced death) or tumor progression results (e.g., tumor weight, growth rate, metastasis, metabolic activity, and survival time with tumor); (III) the fasting therapy should be launched with complete deprivation of food (articles investigating partial calorie restriction (CR) were excluded); (IV) the duration of time for one fasting should be at least 24 hours (articles investigating nightly fasting were excluded); (V) were in language of English.
The quality of included animal studies was assessed according to the ARRIVE guidelines (animal research: reporting in vivo experiments) (https://www.nc3rs.org.uk/arrive-guidelines), which are recommended for quality assessment of animal experiments. The items are based on the presence and description of several important study characteristics, including title, abstract, background, objectives, study design, ethical statement, experimental procedures, experimental animals, sample sizes, allocating animals to experimental groups, housing and husbandry, experimental outcomes, adverse events, statistical method, adverse events, interpretation/scientific implications, conflict of interest, and funding. For human studies, NOS (Newcastle-Ottawa Scale) scoring system (www.ohri.ca/programs/clinical_epidemiology/oxford.asp) was used to assess the quality. Based on the quality of each publication in selection, comparability and exposure, a score with a maximum of nine points was appointed. Articles with six or more of the NOS scores were deemed as high quality and were included in the systematic review.
Data of interest were extracted independently by three authors. A Microsoft Excel sheet was designed to collect the following records: (I) basic information including first author, year of publication, experimental subject, tumor type and tumor inoculation method; (II) intervention strategies including chemotherapy regimens (when the chemotherapy is applied), fasting duration and fasting cycles; (III) outcome measures including chemotherapy side-effects and tumor progression data; and (IV) relevant regulations in pathways including changes in expression of effectors and the following alternations in pathways. Study results that were reported to be “statistically significant” reached P value <0.05 according to the original data.
Results
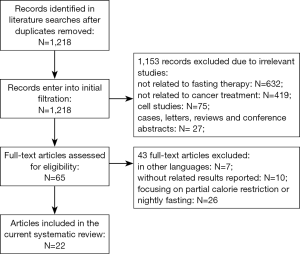
In the initial searches, a total of 1,218 articles were identified after duplicated removed. Then, 1,153 articles were excluded due to irrelevant contents, including 632 studies not related to fasting therapy, 419 studies not related to cancer treatment, 75 studies on cells, and 27 cases, letters and conference abstracts. Among the remaining 65 studies, 43 were excluded after secondary full-text screening, including 7 studies in other languages, 10 studies without related results reported, and 26 studies focusing on partial CR or nightly fasting. Eventually, a total of 22 articles published from 2002 to 2016 were considered as eligible and included in the current systematic review (9-30) (Figure 1).
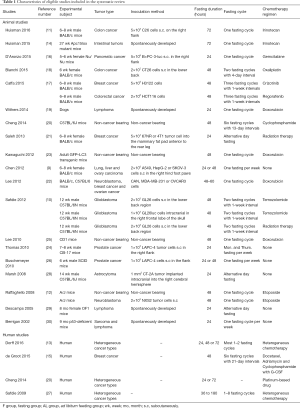
The basic characteristics of the 22 eligible studies are summarized in Table 1. The experimental subject was mice in 18 studies, dogs in one study; as one study with mice experiments also included a preliminary trial on human, a total of four studies evaluated the effects of fasting therapy on cancer patients. For the 19 animal studies, fasting duration of 24 to 72 hours was adopted in most of the studies. Besides experiments using non-cancer bearing models, animal models with heterogeneous tumor types were adopted, including colon cancer, pancreatic cancer, breast cancer, lung cancer, breast cancer, lymphoma, glioblastoma, astrocytoma and sarcoma. All the animal studies were randomized controlled trials, and reported the title, abstract, background, objectives, study design, ethical statement, experimental procedures, experimental animals, sample sizes, allocating animals to experimental groups, experimental outcomes, adverse events, statistical methods, and interpretation/scientific implications. As for the housing environment, 16 of the 19 (84.2%) animal studies mentioned it. The animal studies were in accordance with the ARRIVE guidelines. For the four human studies, as patients with different cancers were included, chemotherapy regimens were heterogeneous. Fasting cycles were also different according to each patient’s clinical circumstances. All the human studies have more than six of the NOS scores.
Full table
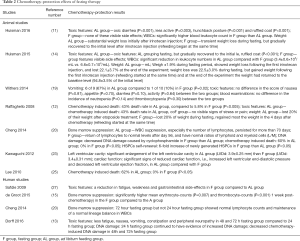
The chemotherapy-protection effects of fasting therapy were summarized in Table 2. Overall, majority of the studies suggested satisfactory chemotherapy-protection effects of fasting therapy, which included reducing toxic features, relieving bone marrow suppression, restoring cardiac function and reducing chemotherapy-induced death. In addition, Dorff (13) and Cheng (20) suggested that, compared with 24-hour fasting group, 72-hour fasting group had more normal hematological examination results, less toxic features, and less DNA damaging. Besides, the only study that reported negative effects of fasting therapy in chemotherapy-protection adopted a fasting duration of 24 hours (19), which was consistent with the aforementioned studies (13,20) and indicated the significance of fasting duration in exerting its protection effects.
Full table
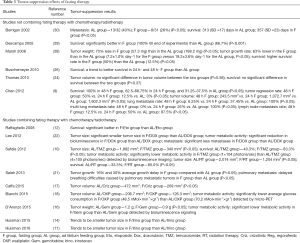
The tumor-suppression effects of fasting therapy were summarized in Table 3. Six studies did not combine fating therapy with chemotherapy, and almost all the studies reported less tumor growth rate, less metastasis and better survival in mice treated with fasting therapy than mice in the ad libitum feeding group. Nine studies combined fasting therapy with chemotherapy/radiotherapy, and seven studies reported significantly more favorable results including smaller tumor size, less metastasis, lower tumor metabolic activity and better survival in the combined treated group than mice only treated with chemotherapy/radiotherapy. The rest two studies found a trend of smaller tumor size in the combined treated group but did not reach significance.
Full table
For the relevant regulations in pathways during fasting therapy, effectors including IGF-1, p-Akt, IRS, BAD, mTOR, p-S6K and Ras that were involved in pI3K-Akt, mTOR and MAPK pathways were found to be down-regulated in mice with fasting therapy (9,16,17,20-22,28). Besides, the proliferation marker Ki-67 (16) and hypoxia-inducible factor (HIF) (28) which promote angiogenesis were down-expressed, while eIF2α (22) which attenuate protein synthesis and apoptosis marker casp-3 and casp-9 (22,28) were up-expressed in tumors of fasting mice. Under microscope inspection, tumor section of fasting mice showed less invasive and destructive features than that in ad libitum feeding mice (9).
Discussion
Nutrient deprivation encompasses several forms of dietary restriction including CR and fasting. CR commonly described a reduction in calorie intake by 20–40%, and it can also refer to more or less strict calorie limitation, or reduced or lack of some particular components of the diet (31,32). Fasting is another form of dietary restriction, which refers to the complete lack of food or calorie intake for a period. The fasting duration usually lasts for 24 to 120 hours, followed by the refeeding period, during which the ad libitum to food was permitted, thus forming a cycle. When fasting protocols contain cycles of fasting, it is called as intermittent fasting (IF). Generally, CR is more studied than fasting in treating tumors, partly due to that a common therapeutic goal in cancer treatment is trying to avoid excess weight loss to counteract wasting syndromes, and that CR which avoided massive fluctuations in dietary intake was initially believed to be more tolerated and causing less side-effects in cancer patients. However, a growing number of recent studies have demonstrated that fasting may be a more advisable choice for nutrient deprivation in these patients, for which the reasons are listed as the followings.
Firstly, gradual and continuous loss of body weight is commonly seen in CR, which could cause the weakening of the immune system (33-35). On the contrary, although the weight loss during fasting was substantial, it was temporary and the weight would regain to the initial level during ad libitum refeeding in the post-fasting period. In fact, no remarkable difference in total food consumption was observed between fasting mice and ad libitum feeding mice in the control group (9,29,30,36), indicating that the efficacy of fasting did not really depend on the calorie deprivation. Secondly, fasting may be more efficient than CR in forming a protection effect. After undergoing CR or fasting for the same time, metabolic parameters such as blood glucose level and IGF-1 level in fasting mice were significantly lower (25,37-39), indicating that fasting is less time-consuming and more efficient than CR. Thirdly, compliance remained an issue in CR because it required the reduction of caloric intake for extended periods of time, whereas fasting therapy could be well implemented and tolerated in cancer patients, although it may be psychologically uncomfortable in some cases (27,40). Therefore, fasting therapy cold avoided the common weight loss in CR, meanwhile having a more efficient effect and a better compliance. Accordingly, we conducted the current systematic review to combine published studies in this field and to comprehensively evaluate the effects of fasting therapy in cancer treatment.
Chemotherapy treatment often relies on the combination of several DNA-damaging drugs such as etoposide, doxorubicin and cyclophosphamide. Although these agents are generally much more toxic to cancer cells than normal cells, the damage to normal tissues is inevitable, possibly leading to intestinal, hematological and systemic toxicities including diarrhea, vomiting, fatigue, myelosuppression, and in some cases chemotherapy interruption and even death (41,42).
In recent studies, pre-chemotherapy fasting was found to have a promising effect on protecting mammalian cells from chemotherapeutic toxicities. Raffaghell reported that mice that have been starved for 48–60 hours showed no visible signs of toxicity under 80 to 110 mg/kg doses of etoposide, which was nearly three times of the maximum allowable concentration of etoposide in humans (30–45 mg/kg). In contrast, the mortality rate of ad libitum feeding mice was over 40%. More interestingly, while the etoposide application caused a 20–40% weight loss in ad libitum feeding mice, the fasting mice gained back most of the weight loss that was lost during fasting in only 4 days after the chemotherapy initiation (12). Similarly, Kawaguchi found that, after doxorubicin treatment, ad libitum feeding mice suffered a more significant cardiac deterioration characterized by enlargement of ventricular cavity and reduced cardiac function compared with mice that was pre-starved for 48 hours, while the fasting itself had no effect on cardiac function in the saline group (23). In addition, consistent results were also reported in other studies that chemotherapy toxic features including lower activity, hunched-back posture, ruffled coat, diarrhea and leukopenia were much more significant in ad libitum feeding mice than fasting mice with a 48- to 72-hour starvation (11,14,20,25).
Apart of the aforementioned mice experiments, the chemotherapy-protection effects were also evaluated in dogs and humans, and moreover, these studies may have indicated the importance of the duration of fasting. As reported by Withers (19), the rates of nausea, diarrhea, lower activity, neutropenia, thrombocytopenia and IGF-1 levels showed no difference between fasting dogs and ad libitum feeding dogs, which were inconsistent with the aforementioned studies. One of the major differences was the fasting duration, which was 24 hours in the study and at least 48 hours in other studies (Table 1), indicating a potential impact of fasting duration on its efficacy. Interestingly, Dorff conducted a study on cancer patients which included three cohorts fasted for 24, 48 and 72 hours before chemotherapy. The study suggested that patients in the 48- or 72-hour fasting group had significantly lower rate of chemotherapy toxicities with decreased DNA damaging compared with the 24-hour fasting group (13). Similar results were also reported by Cheng that normal lymphocyte counts and normal lineage balance of WBCs were observed in cancer patients with 72-hour fasting but not found in those with 24-hour fasting (20). Although species differences between mice and dogs or human beings could influence the outcomes of fasting therapy, these studies still highlight that the 24-hour fasting period may be insufficient to exert the chemotherapy-protection effects.
In addition to chemotherapy-protection effects, it was found that fasting therapy had remarkable effects in inhibiting tumor progression. The tumor size in mice treated with IF alone could be comparable to that in mice underwent two or three cycles of chemotherapy (10,22), which indicated that IF may be as effective as chemotherapeutic agents in delaying tumor growth. When compared to al libitum feeding mice, the tumor size or weight in mice under IF were significantly smaller (Table 3).
The effects of fasting therapy in inhibiting tumor metastasis were also remarkable. Berrigan reported a multiple tumor rate of 26% in mice that underwent IF compared to 40% in ad libitum feeding mice 4 weeks after tumor cell inoculation (30). Similarly, Chen found that the lung metastasis rate was 100% in ad libitum feeding mice and all were multiple lung metastases, whereas the rate was only 6.25% in mice that underwent IF and none had a multiple metastasis (9). As fasting mice trend to have favorable results in tumor growth and metastasis, almost all the included studies reported significantly better survival in mice with fasting therapy alone than ad libitum feeding mice (Table 3).
When fasting therapy was coupled with chemotherapy, the anti-tumor effect of chemotherapeutic drugs was observed to be increased. Among the nine studies combining chemotherapy with fasting therapy on mice, seven studies reported significantly more favorable outcomes including smaller tumor size, less metastases and better survival than those with chemotherapy alone (Table 3). Besides, four studies detected tumor metabolic activity by bioluminescence imaging and micro-PET imaging, all found that the tumor metabolism was significantly lower in combined treatment group (10,16,18,22). In addition to the conventional chemotherapy regimens, the combination of IF with tyrosine kinase inhibitors (TKIs) such as Crizotinib or Regorafenib also could achieve significantly better therapeutic effects (17), suggesting IF as a potential means to enhance the activity of TKIs in clinical practice. Thus, a synergistic effect could be formed by combining chemotherapy with fasting therapy, which would achieve a more satisfactory therapeutic outcome than utilizing either of the individual treatment method alone.
One point that needs to be mentioned is the refeeding protocol during the post-fasting period. All the included studies did not limit the food intake during refeeding. The overfeeding is common in the period and the weight of mice generally would regain to their initial levels. In fact, after completing a whole fasting cycle, the total food consumption between fasting mice and ad libitum feeding mice was similar. Thus, it seems that the effects of fasting therapy were not really dependent on the calorie deprivation. There still lack of study that limits the calorie intake of refeeding to the same as age-matched ad libitum feeding mice, thus preventing the overfeeding. Whether the sufficient calorie supply in the whole fasting therapy cycle is necessary in exerting its effects still remains a question, and it needs to be clarified in future studies, which would help us to further understand the action processes of fasting therapy.
The chemotherapy-protection mechanisms of fasting therapy were partly correlated with the viewpoint that normal cells would rearrange the energy intake into maintenance pathways instead of growth and reproduction during fasting, enhancing resistance to the environment. Fasting was correlated with reduced levels of IGF-1, p-Akt, IRS, BAD, mTOR, p-S6K and Ras, which are effectors involved in several important pathways including PI3K-Akt, mTOR and MAPK pathways (9,16,17,20-22,28). Through this, growth factors and proliferation signals drop during fasting, and normal cells which require these factors and signals for proper growth and reproduction would redistribute the limited resource to maintain survival and turnoff unnecessary energy expenditures including synthesis, growth and proliferation. However, cancer cells are self-sufficient of growth factors due to mutations in proto-oncogenes, which could enable cancer cells to proliferation independently and show no response to the stressed conditions (43).
As effects of chemotherapy drugs were correlated with metabolic activity of cells, fasting mice with lower metabolic levels would suffer less impact of chemotherapy regimens to the normal cells, thus reducing side-effects. Besides, fasting was found to play a crucial role in protecting hematopoietic stem cell from chemotoxicity, promoting its self-renewal and regeneration, and thus reversing the immunosuppression (20). As a result, mice under fasting therapy which had less chemotherapy-induced side effects showed a higher tolerance to chemotherapy regimens, and it seems that higher doses of chemotherapy regimens could be applied if fasting therapy was combined with the chemotherapy. Thus, whether the combination with fasting therapy could raise the upper limits of tolerable chemotherapy doses, and whether the methods could achieve a higher tumor control rate may be promising directions for future researches.
As the normal cells and cancer cells exhibit differential stress resistance to fasting, it is likely that cancer cells are more sensitive to the extreme environments for their inability to timely adjust metabolism pathways. The intratumoral HIF was down-expressed in fasting mice (28), which indicated decreased tumoral angiogenesis processes under fasting condition. Besides, cancer cells in mice with fasting therapy showed significant decreased activity, which was suggested by down-expression of proliferation marker Ki-67 (16), up-expression of eIF-2α that impairs protein synthesis (22), and up-expression of apoptosis marker casp-3 and casp-9 (22,28). In addition, microscopical findings by Chen showed that local tumor nests in ad libitum feeding mice were faster growing with invasive and destructive features, whereas tumor nests in fasting mice underwent pyknosis and shrinking (9), which could further provide morphological evidence of tumor-suppression effects of fasting therapy.
Currently, studies investigating fasting therapy on cancer patients are limited, with only 10 to 20 patients included in each study, and all were in the stage of evaluating safety and chemotherapy-protection effects of fasting therapy (13,15,20,27). The tumor-suppression effects of fasting, e.g., its influence on tumor growth, metastasis and prognosis of patients, have not been evaluated until now.
On the other hand, the appliance of fasting therapy on cancer patients should be with caution and needs to be more firmly established. The patients with diabetics and the very old may should avoid fasting therapy, and whether fasting therapy would reinforce cachexia should also be assessed. Another point that needs to be paid attention is the fasting duration. In the systematic review, we inferred that fasting duration of 24 hours may be insufficient for exerting the chemotherapy protection effects of fasting therapy. However, overlong fasting duration should also be avoided for the possible severe calorie insufficiency and weakening of the body. The length of fasting duration is like a cost-benefit ratio, and exploring an optimal fasting duration may be a promising way for future studies. Moreover, the effects of fasting therapy could be influenced by the tumor types and the applied chemotherapy drugs. Combined with what chemotherapy drugs and treating what tumor types could maximize the effects of fasting therapy are still largely unknown, and it needs to be clarified by future studies.
It needs to be mentioned that studies about the Ramadan fasting is not included in the current systematic review, for it usually lasts for about 12 hours per cycle and the water is also forbidden during fasting, which is not in accordance with our inclusion criteria. However, the influence of Ramadan fasting in cancer patients should be paid attention, for that very few studies have been carried out focusing on this issue (44). Investigating the correlation between Ramadan fasting and cancer treatment not only could provide a new way to further understand the mechanisms of fasting therapy, but also could help physicians to properly advise cancer patients fasting in Ramadan (44).
Overall, fasting therapy may have effective chemotherapy-protection and tumor-suppression effects in mammals, and may be a feasible option in cancer treatment to further improve prognosis. Future well-designed clinical trials are still needed before fasting therapy could be used in standard practice.
Acknowledgments
The authors gratefully acknowledge the staff in the Department of Oncology and Evidence-Based Medicine Center, West China Hospital, Sichuan University.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.03.35). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Siegel RL, Ward EM, et al. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev 2016;25:16-27. [Crossref] [PubMed]
- Chen HM, Tsai CM, Wu YC, et al. Randomised controlled trial on the effectiveness of home-based walking exercise on anxiety, depression and cancer-related symptoms in patients with lung cancer. Br J Cancer 2015;112:438-45. [Crossref] [PubMed]
- Hayes BD, Brady L, Pollak M, et al. Exercise and Prostate Cancer: Evidence and Proposed Mechanisms for Disease Modification. Cancer Epidemiol Biomarkers Prev 2016;25:1281-8. [Crossref] [PubMed]
- Friedenreich CM, Neilson HK, O'Reilly R, et al. Effects of a High vs Moderate Volume of Aerobic Exercise on Adiposity Outcomes in Postmenopausal Women: A Randomized Clinical Trial. JAMA Oncol 2015;1:766-76. [Crossref] [PubMed]
- Flynn PM, Betancourt H, Ormseth SR. Culture, emotion, and cancer screening: an integrative framework for investigating health behavior. Ann Behav Med 2011;42:79-90. [Crossref] [PubMed]
- Prentice RL, Thomson CA, Caan B, et al. Low-fat dietary pattern and cancer incidence in the Women's Health Initiative Dietary Modification Randomized Controlled Trial. J Natl Cancer Inst 2007;99:1534-43. [Crossref] [PubMed]
- Baade PD, Youlden DR, Krnjacki LJ. International epidemiology of prostate cancer: geographical distribution and secular trends. Mol Nutr Food Res 2009;53:171-84. [Crossref] [PubMed]
- Wang A, Huen SC, Luan HH, et al. Opposing Effects of Fasting Metabolism on Tissue Tolerance in Bacterial and Viral Inflammation. Cell 2016;166:1512-25.e12. [Crossref] [PubMed]
- Chen X, Lin X, Li M. Comprehensive modulation of tumor progression and regression with periodic fasting and refeeding circles via boosting IGFBP-3 loops and NK responses. Endocrinology 2012;153:4622-32. [Crossref] [PubMed]
- Safdie F, Brandhorst S, Wei M, et al. Fasting enhances the response of glioma to chemo- and radiotherapy. PLoS One 2012;7:e44603 [Crossref] [PubMed]
- Huisman SA, de Bruijn P, Ghobadi Moghaddam-Helmantel IM, et al. Fasting protects against the side effects of irinotecan treatment but does not affect anti-tumour activity in mice. Br J Pharmacol 2016;173:804-14. [Crossref] [PubMed]
- Raffaghello L, Lee C, Safdie FM, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A 2008;105:8215-20. [Crossref] [PubMed]
- Dorff TB, Groshen S, Garcia A, et al. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer 2016;16:360. [Crossref] [PubMed]
- Huisman SA, Bijman-Lagcher W, IJzermans JN, et al. Fasting protects against the side effects of irinotecan but preserves its anti-tumor effect in Apc15lox mutant mice. Cell Cycle 2015;14:2333-9. [Crossref] [PubMed]
- de Groot S, Vreeswijk MP, Welters MJ, et al. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: a randomized pilot study. BMC Cancer 2015;15:652. [Crossref] [PubMed]
- D’Aronzo M, Vinciguerra M, Mazza T, et al. Fasting cycles potentiate the efficacy of gemcitabine treatment in in vitro and in vivo pancreatic cancer models. Oncotarget 2015;6:18545-57. [Crossref] [PubMed]
- Caffa I, D'Agostino V, Damonte P, et al. Fasting potentiates the anticancer activity of tyrosine kinase inhibitors by strengthening MAPK signaling inhibition. Oncotarget 2015;6:11820-32. [Crossref] [PubMed]
- Bianchi G, Martella R, Ravera S, et al. Fasting induces anti-Warburg effect that increases respiration but reduces ATP-synthesis to promote apoptosis in colon cancer models. Oncotarget 2015;6:11806-19. [Crossref] [PubMed]
- Withers SS, Kass PH, Rodriguez CO Jr, et al. Fasting Reduces the Incidence of Delayed-Type Vomiting Associated with Doxorubicin Treatment in Dogs with Lymphoma. Transl Oncol 2014; [Epub ahead of print]. [Crossref] [PubMed]
- Cheng CW, Adams GB, Perin L, et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell 2014;14:810-23. [Crossref] [PubMed]
- Saleh AD, Simone BA, Palazzo J, et al. Caloric restriction augments radiation efficacy in breast cancer. Cell Cycle 2013;12:1955-63. [Crossref] [PubMed]
- Lee C, Raffaghello L, Brandhorst S, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med 2012;4:124ra27 [Crossref] [PubMed]
- Kawaguchi T, Takemura G, Kanamori H, et al. Prior starvation mitigates acute doxorubicin cardiotoxicity through restoration of autophagy in affected cardiomyocytes. Cardiovasc Res 2012;96:456-65. [Crossref] [PubMed]
- Thomas JA 2nd, Antonelli JA, Lloyd JC, et al. Effect of intermittent fasting on prostate cancer tumor growth in a mouse model. Prostate Cancer Prostatic Dis 2010;13:350-5. [Crossref] [PubMed]
- Lee C, Safdie FM, Raffaghello L, et al. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res 2010;70:1564-72. [Crossref] [PubMed]
- Buschemeyer WC 3rd, Klink JC, Mavropoulos JC, et al. Effect of intermittent fasting with or without caloric restriction on prostate cancer growth and survival in SCID mice. Prostate 2010;70:1037-43. [Crossref] [PubMed]
- Safdie FM, Dorff T, Quinn D, et al. Fasting and cancer treatment in humans: A case series report. Aging (Albany NY) 2009;1:988-1007. [Crossref] [PubMed]
- Marsh J, Mukherjee P, Seyfried TN. Akt-dependent proapoptotic effects of dietary restriction on late-stage management of a phosphatase and tensin homologue/tuberous sclerosis complex 2-deficient mouse astrocytoma. Clin Cancer Res 2008;14:7751-62. [Crossref] [PubMed]
- Descamps O, Riondel J, Ducros V, et al. Mitochondrial production of reactive oxygen species and incidence of age-associated lymphoma in OF1 mice: effect of alternate-day fasting. Mech Ageing Dev 2005;126:1185-91. [Crossref] [PubMed]
- Berrigan D, Perkins SN, Haines DC, et al. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis 2002;23:817-22. [Crossref] [PubMed]
- Omodei D, Fontana L. Calorie restriction and prevention of age-associated chronic disease. FEBS Lett 2011;585:1537-42. [Crossref] [PubMed]
- Chung KW, Kim DH, Park MH, et al. Recent advances in calorie restriction research on aging. Exp Gerontol 2013;48:1049-53. [Crossref] [PubMed]
- Kristan DM. Calorie restriction and susceptibility to intact pathogens. Age (Dordr) 2008;30:147-56. [Crossref] [PubMed]
- Racette SB, Weiss EP, Villareal DT, et al. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci 2006;61:943-50. [Crossref] [PubMed]
- Kristan DM. Chronic calorie restriction increases susceptibility of laboratory mice (Mus musculus) to a primary intestinal parasite infection. Aging Cell 2007;6:817-25. [Crossref] [PubMed]
- Varady KA, Hellerstein MK. Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr 2007;86:7-13. [PubMed]
- Heilbronn LK, Smith SR, Martin CK, et al. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr 2005;81:69-73. [PubMed]
- Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol 2007;42:709-12. [Crossref] [PubMed]
- Barger JL, Kayo T, Vann JM, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One 2008;3:e2264 [Crossref] [PubMed]
- Deshmukh S, Phillips BG, O'Dorisio T, et al. Hormonal responses to fasting and refeeding in chronic renal failure patients. Am J Physiol Endocrinol Metab 2005;288:E47-55. [Crossref] [PubMed]
- Kayl AE, Meyers CA. Side-effects of chemotherapy and quality of life in ovarian and breast cancer patients. Curr Opin Obstet Gynecol 2006;18:24-8. [Crossref] [PubMed]
- Perrino C, Schiattarella GG, Magliulo F, et al. Cardiac side effects of chemotherapy: state of art and strategies for a correct management. Curr Vasc Pharmacol 2014;12:106-16. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70. [Crossref] [PubMed]
- Bragazzi NL, Briki W, Khabbache H, et al. Ramadan Fasting and Patients with Cancer: State-of-the-Art and Future Prospects. Front Oncol 2016;6:27. [Crossref] [PubMed]