
Initial experience of intraoperative radiotherapy as tumour bed boost after neoadjuvant chemotherapy in breast cancer patients
Introduction
The incidence of breast cancer is steadily increasing worldwide, it accounts for 23% of all cancers in women. Breast cancer is the most common malignant neoplasm in women and a frequent cause of death in Europe (1,2). The treatment of breast cancer including surgery, chemotherapy, anti-hormonal therapy, targeted therapy, and radiation heavily depends on both the characteristics of the tumour, such as stage, presence of hormone receptors, and other pathological parameters, as well as general patient characteristics, such as age, comorbidities, personal/individual preferences, etc. Early breast cancer is usually treated with breast conserving surgery (BCS) involving the wide excision of the tumour (typically along with some of the surrounding healthy tissue) and frequently sentinel lymph node biopsy, followed by external beam whole breast radiotherapy (EBRT) (2). This conventional radiotherapy after surgery leads to a significant decrease in local relapse (3,4), while an additional radiation dose delivered as a boost to the tumour bed may further decrease the rate of local tumour recurrence (5-9).
For the tumour bed boost there are different techniques including external beam radiotherapy and intraoperative radiotherapy (IORT) with electrons or low energy X-rays. IORT itself can also be delivered with different techniques including conventional linear accelerators, mobile devices generating fast electrons or low-energy X-rays (10-12). The mobile device Intrabeam® (Carl Zeiss Meditec, Oberkochen, Germany), a miniature X-ray source, has been used for tumor bed boost radiation during BCS for more than 10 years at the University Medical Centre Mannheim. After the tumour resection the wound cavity is treated immediately with low-energy X-rays (50 kV) (11). IORT has proven beneficial as one option of applying the boost because it safely covers the tumour bed without conferring negative effects on the vicinity whereas EBRT contains the risk of missing the intended boost target volume, i.e., the tumour bed (geographic miss), on top of the delay between surgery and radiation therapy (temporal miss) (3,11).
Several studies suggest that although available data are still limited, the efficacy and safety of an IORT boost is not inferior to a conventional boost (2,12,13). The relapse rate appears at least similar in women treated with IORT boost and women receiving conventional external boost (2,12). In the treatment of breast cancer IORT boost seems to be a promising alternative because of the reduced interval between surgery and radiotherapy (2,9,11,12). In this regard, Wenz and colleagues could demonstrate that IORT boost with 50 kV X-rays is a safe alternative for treatment (12) covered by official guidelines (14). Apart from the low rates of complication in regard to short-term results of BCS and IORT, IORT itself has low chronic skin toxicity rates and excellent results regarding acute and late toxicity and local control (12,15).
The necessity of treatment with chemotherapy depends on the stage and biology of the tumour, including tumour size and grading, the presence of hormone receptors, and the lymph node involvement. In most cases, chemotherapy is still given after surgery. However, in an increasing number of patients chemotherapy is administered before the surgical removal of the tumour. This primary or neoadjuvant chemotherapy (nCHT) is aimed to minimise the primary tumour size thereby enabling the surgeon to perform BCS less destructive. Furthermore tumour response to neoadjuvant chemotherapy is a reliable prognostic factor and offers insights into the biology of the malignancy. Neoadjuvant chemotherapy is also a standard treatment option in inflammatory breast cancer, for initially inoperable tumours (T4) and for triple negative breast cancer (TNBC) (16-18).
Reviewing the literature there are no studies dealing with the toxicity of IORT boost after neoadjuvant chemotherapy. The aim of this investigation is to report initial experience with IORT after BCS in patients after completion of nCHT.
Methods
Between August 2005 and February 2012 a total of 13 women, who had completed neoadjuvant chemotherapy, were treated with an IORT boost using the mobile device Intrabeam® during BCS and a postoperative conventional EBRT at the University Medical Centre Mannheim, Germany. One woman suffered from bilateral breast cancer; therefore BCS with IORT was performed bilaterally.
The method and technical procedure of IORT have been published before and are described extensively in several publications (11,12,15).
The Intrabeam® system is composed of a miniature (1.6 kg) X-ray source having a probe of 10cm length and 3.2 mm diameter, a set of spherical applicators from 1.5 to 5.0 cm in diameter, a carrier system and a control unit. Accelerated electrons aimed on a gold target produce a spherical radiation field with an isotropic dose distribution around the tip of the probe. Using this method low energy X-rays (50 kV) are generated. Due to the steep dose falloff, this mobile device can be used in almost unmodified operating rooms. Prior to use, mechanical stability, dose rate and homogeneity of the emitted radiation are checked in detail. The patients received a perioperative intravenous antibiotic treatment with 2 g cefazolin. After informed consent the surgical and radiotherapeutic procedures were performed as a standardised operating procedure. Histological findings were assessed at the Department of Pathology at the University Medical Centre Mannheim.
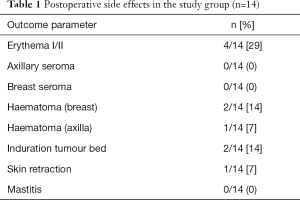
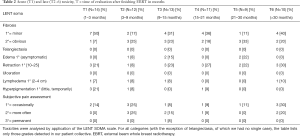
Demographic and surgical parameters as stated below were analysed retrospectively. Clinical outcomes as well as cosmetic results were evaluated every day during the first week after surgery (postoperative follow-up, Table 1) as well as during regularly scheduled follow-up visits (acute and late toxicity assessment, Table 2). Acute toxicities were defined as occurring between 1 to 3 months. Adverse side effects after more than 3 months were considered late toxicities, with final evaluation of late toxicities being performed at 31–41 months post intervention. Toxicities were assessed using the Common Terminology Criteria for Adverse Events (CTCAE) (19), the LENT-SOMA scale was applied where appropriate. Other findings were documented. This findings include: haematoma/suggillation, palpable seroma, mastitis, the necessity of therapeutic application of antibiotics, induration of the tumour bed, retraction of the scar, postoperative fever, pre- and post-operative blood count and the usage of postoperative pain relievers.
Full table
Full table
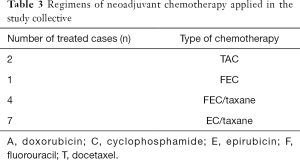
The most frequently applied chemotherapy regimen was four cycles of EC (90 mg/m2 epirubicin, 600 mg/m2 cyclophosphamide) followed by four cycles of Docetaxel (100 mg/m2). A detailed description of the different chemotherapy protocols is depicted in Table 3. Pathological complete response (pCR) was diagnosed in 4 cases (29%, 4/14).
Full table
Mean duration of surgery was 128 minutes with mean duration of irradiation of 29 minutes. The most frequently used Intrabeam® applicator was 4.5 cm in diameter. All procedures were conducted as an irradiation with a dose prescription of 20 Gy on the applicator surface. In nine cases a sentinel node biopsy (SNB) had been performed. Axillary dissection in combination with BCS and IORT was performed in five cases.
Antihormonal therapy and CHT were recommended and administered according to tumour board decisions based on respective guidelines, which resulted in 9 patients (64%, 9/14) receiving antihormonal therapy and no patient being subjected to adjuvant CHT (0%, 0/14). One patient was Her2-neu positive and therefore received trastuzumab in congruence with current guidelines.
Following the combination of IORT and BCS, the therapeutic regimen was subsequently augmented by EBRT. Whole breast radiotherapy was given five times per week with 1.8–2.0 Gy per fraction (1.8 Gy n=1, 2.0 Gy n=12) in supine position with a dedicated linear accelerator [Elekta (R)]. Mean dose to the whole breast was 46.0 Gy (range, 0–50.4 Gy).
All data were collected in an Excel™ (Microsoft Corporation, Redmond, Seattle, USA) datasheet. Quantitative data were presented as mean, median and range, qualitative data as frequencies.
Results
Our study analysed acute and late follow-up data of 14 breast cancers in 13 patients with BCS and IORT after neoadjuvant chemotherapy. Age of the 13 included women ranged from 32 to 68 years (mean =50 years). Of note, one patient was included in our study despite having metastases (liver, bone) at the time of initial diagnosis rendering her unable to receive neoadjuvant chemotherapy in curative intent. Nevertheless, we included her in our pilot study as she was treated with (palliative) chemotherapy followed by treatment of IORT boost during BCS.
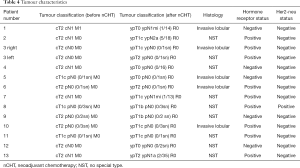
In most cases chemotherapy was applied without major side effects, but in 5 cases the following side effects occurred during chemotherapy: febrile neutropenia (3/14 cases; 21%), anaemia (3/14 cases; 21%) and neuropathy (1/14 cases; 7%). The mean tumour size before the start of chemotherapy ranged between 1.2 and 5 cm. After chemotherapy the size of the largest tumor was 2 cm. A detailed description of tumour characteristics and histological findings is shown in Table 4.
Full table
In general the observed acute toxicity after IORT was low and all breast conserving surgeries could be performed without major intraoperative complications. In 5 cases (5/14, 36%) a second surgical intervention for acceptable resection margins was necessary. In one case (1/14; 7%) a revision was necessary because of axillary bleeding. No patient suffered from postoperative fever, which was defined as elevated body temperature higher than 38.5 °C measured with a tympanic thermometer.
The most frequent immediate side effect after BCS/IORT was erythema grade I or II occurring in 4 breasts (4/14; 29%). None of the women suffered from palpable, clinically relevant breast or axillary seroma. A detailed description of the immediate postoperative side effects is given in Table 1.
Late follow-up analysis (see Table 2) could be performed in 10 patients with a median follow-up of 40 months (range 31–41 months). Six of these patients (6/10 cases, 60%) presented with grade I or II fibrosis within the tumour bed during this follow-up. No grade III fibrosis was seen. Three patients (3/10 cases, 30%) had developed skin retraction. Five patients (5/10, 50%) complained about pain: three of these patients suffered from infrequent pain (G1), while two patients reported the pain to be more frequent (G2 pain). None of the patients suffered from pain permanently (G3 pain). All other toxicities that can be assessed with the LENT SOMA scale (edema of the breast, ulceration, lymphedema of the arm, telangiectasia or hyperpigmentation) could not be detected in the late follow-up of these ten patients (a detailed description of late follow-up data can be obtained from Table 2).
During the follow-up, there was only one patient with a local recurrence in the breast. This patient was the youngest woman in our series, aged 32, having an advanced tumor size (ypT2), poor response to neoadjuvant chemotherapy, lymphangioinvasion and a highly aggressive biology (G3).
Discussion
Breast cancer treatment has seen an impressive evolution in the past decades as therapeutic options and regimens have been augmented by the addition and combination of new drugs (modified CHT, antihormonal agents and antibody therapies) on the one hand and fascinating developments in radiotherapy on the other hand. In regard to the latter, several studies could demonstrate that efficacy and safety of an IORT boost are not inferior to a conventional treatment, establishing this modality as a beneficial alternative for treatment in breast cancer patients (11,15,20-22). Neoadjuvant chemotherapy has been standard therapy in inflammatory breast cancer and for treatment of initially inoperable tumors (T4). Nowadays it is recommended as primary treatment in a steadily increasing number of clinical situations with breast cancer such as triple negative disease (16-18).
However, in light of these therapeutic developments, we screened the literature for combinations of both treatment regimens (nCHT and IORT). This review of current literature on IORT and nCHT revealed that there is currently only one seminal study examining the results and outcome of IORT boost after neoadjuvant chemotherapy (23). In this important study, Fastner and colleagues present first evidence that IORT with electrons (IOERT) may constitute a beneficial treatment option after completion of nCHT in locally advanced malignancy. They confirm the role of IORT as a highly effective boost strategy in terms of disease control and survival (23).
Therefore the purpose of this retrospective study was to evaluate and report first clinical experience of IORT (with 50 kV X-rays) during BCS in patients who had previously completed nCHT. As previously mentioned, one of the patients in our cohort did not meet the criteria of nCHT, but was rather primarily subjected to palliative chemotherapy (due to metastases of liver and bone). In this special case however, success of palliative chemotherapy led to the decision to offer combination of simultaneous IORT boost during surgical treatment (BCS) rendering the observations from this case applicable for the purposes and hypotheses of this retrospective pilot study.
In general and as a note of caution not to fall for this potential bias, it can be argued that any differences in morbidity (either local complications/observations such as skin retraction and hematoma or systemic complications) in patients with nCHT and BCS with IORT—when compared to previous IORT-studies—might be attributable to a potentially increased tumour burden as well as more advanced cancer stages. Therefore, they may not reflect an actual increase in nCHT-IORT-regimen associated morbidity. Nevertheless, adverse events after the combination of these procedures have to be analysed very thoroughly.
One of the most frequent acute complications of breast cancer surgery itself is seroma. The literature reveals that rates for postoperative seroma vary between 9.2% (3) and 19.2% (24). A previous study on postprocedural seroma in patients treated with BCS and IORT revealed no increase of clinically relevant seromas as compared to conventional therapy (25). Interestingly, our small sample group of 14 cases did not show any palpable acute seroma suggesting that neoadjuvant chemotherapy followed by BCS/IORT does not lead to an increase in clinically relevant breast or axillary seroma.
The published acute complications in IORT-treated patients include hematoma (breast and axilla), erythema, induration and retraction of the scar (1). The rates of these complications were similar in our cohort as compared to previous studies that dealed with BCS/IORT alone or patients who had nCHT and BCS without IORT (1,26,27).
Available literature on cosmetic outcome and toxicity after BCS followed by conventional radiotherapy and BCS combined with IORT demonstrates similar results (15). The frequency and severity of fibrosis in our group of neoadjuvantly treated patients receiving BCS/IORT did not exceed these previous observations providing a first hint that IORT after nCHT is safe and feasible in regard to cosmetic results.
It is well known that late effects are especially important because of their impact on patients’ quality of life (28). Several studies have looked into late morbidity and impairment from radiotherapy. Welzel et al. published that IORT patients have a good quality of life (28). Our current data suggest that neoadjuvant treatment of breast cancer before IORT and BCS does not confer additional impairment in quality of life. In a previous study by Wetzel et al., 43% of patients after EBRT and IORT in combination with BCS were reported to have pain issues during long-term follow-up (28). These results are similar to our observations in patients who had received nCHT before undergoing BCS and IORT.
During follow-up, only one patient was diagnosed with local recurrence in the breast. Not only was this patient the youngest woman in our cohort (32 years of age), but she suffered from highly aggressive tumour biology (G3) and poor response to nCHT in combination with lymphangioinvasion and advanced tumour size (ypT2). As young women with highly aggressive tumour biology are known to be at increased risk of adverse outcome, this observation of local relapse is not surprising and emphasizes the need for close follow up in this high risk group.
In summary, acute and late data from this case series does not provide evidence of a clinically relevant increase of toxicity or adversely affected outcomes in patients treated with a combination of nCHT and BCS/IORT.
However, there are several limitations to our study. First of all, the sample number of our study is very small. Therefore our data must be interpreted extremely cautiously and can only provide a preliminary basis on which future studies with larger sample sizes will have to definitely establish safety after nCHT. In this context, we would like to encourage all groups having experience with nCHT and IORT to publish their data.
It is well known that nCHT may increase the risk of secondary surgical procedures—this is commonly attributed to impaired reliability of intraoperative pathological assessment of the resected material. Consequently, the high rate of surgical re-interventions for complete tumour resection in our collective (5/14, 36% of patients) definitely led to partial resection of IORT-treated tumour bed and may therefore have significantly affected the results. While this resection of irradiated tissue may potentially have caused reductions of particular follow-up complications (e.g., fibrosis and seroma), other problems will potentially have increased due to additional surgery (e.g., pain). Even more important is the fact, that this partial resection of the irradiated tumour bed may potentially influence the risk of local relapse. Therefore this caveat must always be considered when planning a combination of nCHT and IORT.
Conclusions
First data suggest that IORT after nCHT is feasible and relatively safe. The acute and late follow-up did not show major toxicities. The potential implications of the high reexcision rate in our collective have to be taken into consideration. Due to the limited size of our study further research is urgently required to define the role of an IORT boost in breast cancer patients after neoadjuvant systemic therapy.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Carl Zeiss Meditec supports radiobiological research in the Department of Radiation Oncology (to E Sperk and F Wenz). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of the Medical Faculty Mannheim, Heidelberg University and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. As this was a retrospective, chart review study, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tuschy B, Berlit S, Romero S, et al. Clinical aspects of intraoperative radiotherapy in early breast cancer: short-term complications after IORT in women treated with low energy x-rays. Radiat Oncol 2013;8:95. [Crossref] [PubMed]
- Ruano-Ravina A, Cantero-Muñoz P, Eraso Urién A. Efficacy and safety of intraoperative radiotherapy in breast cancer: a systematic review. Cancer Lett 2011;313:15-25. [Crossref] [PubMed]
- Kraus-Tiefenbacher U, Scheda A, Steil V, et al. Intraoperative radiotherapy (IORT) for breast cancer using the Intrabeam system. Tumori 2005;91:339-45. [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [Crossref] [PubMed]
- Baum M, Vaidya JS, Mittra I. Multicentricity and recurrence of breast cancer. Lancet 1997;349:208. [Crossref] [PubMed]
- Machtay M, Lanciano R, Hoffman J, et al. Inaccuracies in using the lumpectomy scar for planning electron boosts in primary breast carcinoma. Int J Radiat Oncol Biol Phys 1994;30:43-8. [Crossref] [PubMed]
- Benda RK, Yasuda G, Sethi A, et al. Breast boost: are we missing the target? Cancer 2003;97:905-9. [Crossref] [PubMed]
- Sedlmayer F, Rahim HB, Kogelnik HD, et al. Quality assurance in breast cancer brachytherapy: geographic miss in the interstitial boost treatment of the tumor bed. Int J Radiat Oncol Biol Phys 1996;34:1133-9. [Crossref] [PubMed]
- Sedlmayer F, Reitsamer R, Fussl C, et al. Boost IORT in Breast Cancer: Body of Evidence. Int J Breast Cancer 2014;2014:472516 [Crossref] [PubMed]
- Blank E, Kraus-Tiefenbacher U, Welzel G, et al. Single-center long-term follow-up after intraoperative radiotherapy as a boost during breast-conserving surgery using low-kilovoltage x-rays. Ann Surg Oncol 2010;17:352-8. [Crossref] [PubMed]
- Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet 2010;376:91-102. [Crossref] [PubMed]
- Wenz F, Welzel G, Blank E, et al. Intraoperative radiotherapy as a boost during breast-conserving surgery using low-kilovoltage X-rays: the first 5 years of experience with a novel approach. Int J Radiat Oncol Biol Phys 2010;77:1309-14. [Crossref] [PubMed]
- Eldredge-Hindy HB, Rosenberg AL, Simone NL. Intraoperative radiotherapy for breast cancer: the lasting effects of a fleeting treatment. Int J Breast Cancer 2014;2014:214325 [Crossref] [PubMed]
- Sedlmayer F, Sautter-Bihl ML, Budach W, et al. DEGRO practical guidelines: radiotherapy of breast cancer I: radiotherapy following breast conserving therapy for invasive breast cancer. Strahlenther Onkol 2013;189:825-33. [Crossref] [PubMed]
- Sperk E, Welzel G, Keller A, et al. Late radiation toxicity after intraoperative radiotherapy (IORT) for breast cancer: results from the randomized phase III trial TARGIT A. Breast Cancer Res Treat 2012;135:253-60. [Crossref] [PubMed]
- Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533-46. [Crossref] [PubMed]
- Harbeck N, Thomssen C, Gnant M, St. . Gallen 2013: brief preliminary summary of the consensus discussion. Breast Care (Basel) 2013;8:102-9. [Crossref] [PubMed]
- Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast 2014;23:489-502. [Crossref] [PubMed]
- Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 2014;106. [PubMed]
- Merrick HW 3rd, Battle JA, Padgett BJ, et al. IORT for early breast cancer: a report on long-term results. Front Radiat Ther Oncol 1997;31:126-30. [Crossref] [PubMed]
- Mussari S, Sabino Della Sala W, Busana L, et al. Full-dose intraoperative radiotherapy with electrons in breast cancer. First report on late toxicity and cosmetic results from a single-institution experience. Strahlenther Onkol 2006;182:589-95. [Crossref] [PubMed]
- Fastner G, Sedlmayer F, Merz F, et al. IORT with electrons as boost strategy during breast conserving therapy in limited stage breast cancer: long term results of an ISIORT pooled analysis. Radiother Oncol 2013;108:279-86. [Crossref] [PubMed]
- Fastner G, Reitsamer R, Ziegler I, et al. IOERT as anticipated tumor bed boost during breast-conserving surgery after neoadjuvant chemotherapy in locally advanced breast cancer--results of a case series after 5-year follow-up. Int J Cancer 2015;136:1193-201. [Crossref] [PubMed]
- Waljee JF, Hu ES, Newman LA, et al. Predictors of breast asymmetry after breast-conserving operation for breast cancer. J Am Coll Surg 2008;206:274-80. [Crossref] [PubMed]
- Kraus-Tiefenbacher U, Welzel G, Brade J, et al. Postoperative seroma formation after intraoperative radiotherapy using low-kilovoltage X-rays given during breast-conserving surgery. Int J Radiat Oncol Biol Phys 2010;77:1140-5. [Crossref] [PubMed]
- Waljee JF, Newman LA. Neoadjuvant systemic therapy and the surgical management of breast cancer. Surg Clin North Am 2007;87:399-415. ix. [Crossref] [PubMed]
- Panhofer P, Ferenc V, Schütz M, et al. Standardization of morbidity assessment in breast cancer surgery using the Clavien Dindo Classification. Int J Surg 2014;12:334-9. [Crossref] [PubMed]
- Welzel G, Hofmann F, Blank E, et al. Health-related quality of life after breast-conserving surgery and intraoperative radiotherapy for breast cancer using low-kilovoltage X-rays. Ann Surg Oncol 2010;17:359-67. [Crossref] [PubMed]


