
A novel functional indel polymorphism within long non-coding RNAs growth arrest specific 5 conferred risk for cervical squamous cell carcinoma in Chinese Han populations
Introduction
Cervical carcinoma is one of the most common malignancies in women worldwide (1). Over 527,600 newly diagnosed cases and 265,700 deaths were attributed to cervical carcinoma in year 2012, with developing countries such as China bearing the major portion burden (2). Cervical squamous cell carcinoma (CSCC) accounts for over 80% of cervical carcinoma and its underlying mechanism is yet to be elucidated. The genetics of CSCC is a highly researched area in recent decades (3). The availability of technologies for genome-wide association study (GWAS) greatly has accelerates the process of disease susceptible loci discovery, and mounting number of susceptible loci for CSCC has been identified since the first application of GWAS on CSCC (4). Because some loci with minor allele frequency less than 5% are not enrolled in GWAS and other disadvantages (5), classical candidate gene approach still contributes to susceptible loci discovery.
Long non-coding RNAs (lncRNAs) are non-coding RNAs with nucleotides longer than 200 base-pairs, and they are found to be key regulators of gene activity (6). Growing evidence demonstrates that lncRNAs are key players in cervical carcinogenesis (7). As one of the lncRNAs, growth arrest specific 5 (GAS5) was formerly identified as anti-oncogenic gene in multiple solid tumors including CSCC (8). Cao and his colleagues identified that GAS5 was a novel biomarker for CSCC progression, its low expression was correlated with poor prognosis (9), Chang and his colleagues found the similar finding in hepatocellular carcinoma (10). However, the role of GAS5 in hepatocellular carcinoma was recognized as proto-oncogenic, it is controversial based on previous identification (11).
A recent research reported that an indel polymorphism (rs145204276) within GAS5 was associated with hepatocellular carcinoma (11), and functional assay confirmed that the polymorphism could influence the methylation status of promoter region for GAS5. The indel polymorphism (rs145204276), residing in the promoter region, could change the methylation status and change the transcription activity. To date, the association between the indel polymorphism (rs145204276) and CSCC risk is still to be clarified. Based on the possible role of GAS5 in CSCC and evidence from hepatocellular carcinoma model, we performed the two-stage case-control investigation in Chinese Han populations to evaluate the association between the rs145204276 polymorphism and CSCC susceptibility. Further in vivo and in vitro assays were implemented to confirm the current finding. The current study is the first report on association of rs145204276 polymorphism with CSCC risk, and it would help to give an insight into roles of polymorphisms within lncRNAs in CSCC pathogenesis.
Methods
Enrolled subjects
The present enrolled CSCC group consisted of 423 histopathologically confirmed cases diagnosed, hospitalized and treated in the affiliated hospital of Xuzhou Medical University from January 2011 to May 2016 and 497 CSCC cases with similar conditions as Xuzhou group from Shehong Hospital of Traditional Chinese Medicine. All cases received none medication before sampling. Clinical tumor stages were judged according to a modified American Joint Committee on Cancer (AJCC) and International Federation of Gynecology and Obstetrics (FIGO). A total of 515 cancer-free controls from Xuzhou center and 503 cancer-free controls from Shehong were individuals selected from local routine physical surveys in Xuzhou and Shehong respectively. Because the samples and information in Xuzhou center were firstly collected, and samples and information in Shehong center were secondly collected, we took subjects from Xuzhou as the discovery set and Shehong counterpart as validation set. Additional tumor tissues from 68 patients that diagnosed as CSCC were gathered from January 2011 to May 2016. The 68 patients is a part of 423 cases enrolled in Xuzhou. The investigation conformed to the Declaration of Helsinki, all subjects recruited were Han Chinese with non-kinship, and each subject wrote confirmed consent for the investigation. Ethical approval for the present investigation was obtained from the Ethical Committee of the affiliated hospital of Xuzhou Medical University and Shehong Hospital of Traditional Chinese Medicine.
DNA isolation and genotyping
Genomic DNA was isolated from peripheral venous blood with Genomic DNA purification kit (Qiagen). Amplification of target DNA fragment with the indel and allelic discrimination was performed with PCR, Native-PAGE electrophoresis and silver staining method as previously reported method (12).
RT-qPCR
Total RNA was isolated from fresh tissue samples with RNA isolation kit (Qiagen).
The cDNA generation and PCR primers were identical with the former study and amplification protocol and reaction system was performed as previously reported (11). The 2−ΔΔCT algorithm was applied to calculate the GAS5 expression levels for different groups (13).
Constructs, cell culture and luciferase assay
The fragment about 300 base-pairs including the insert or deletion allele of rs145204276 in the upstream of GAS5 transcription start point was directly synthesized by Sangon Company (Shanghai). Then the wildtype (ins/ins homozygote) and mutant amplicons (del/del homozygote) were cloned into pGL3-basic (Promega) at the common Hind III and Bgl II restriction enzyme cutting sites respectively and finally named pGL3-WT (ins/ins homozygote) and pGL3-M T (del/del homozygote). The sequence orientation was confirmed by direct sequencing.
Cell lines including H8, SiHa and CaSki were cultured in Roswell Park Memorial Institute media 1640 added with 10% fetal bovine serum and 1% penicillin-streptomycin at 37 °C with 5% CO2 in cell culture box (Thermofisher).
Cells were seeded at a density of 2.5×106 cells/well in 6-well plates (Corning). After 16 hours, cells were transfected by lipofectamine 2000 (Invitrogen) conformed to manufacturer’s protocol. In each well, 500 ng pGL3-WT or pGL3-MT and 50 ng pRL-TK vector (Promega) were cotransfected. pGL3-basic vector with none load was seen as the current negative control. Twenty-four hours after transient transfection, cells were harvested and treated with the protocol from Dual-Luciferase® Reporter Assay System kit (Promega).
Statistical analysis
The difference of age distribution of cases and controls was analyzed by Student’s t-test, frequency difference of tobacco consumption status and menopausal status was compared with chi-square test. Genotypic frequency difference in control group was measured by Hardy-Weinberg equilibrium with chi-square test. Method of genotypic and allelic frequency for comparison of CSCC cases with controls was chi-square test. Ptrend value in Table 1 was derived from linear-by-linear association for chi-square test in crosstabs, it was used in categorical data analysis when the aim is to assess for the presence of an association between a variable with two categories (presence or absence of CSCC risk) and a variable with three categories (rs531564 polymorphism with ins/ins, ins/del and del/del genotype). Unconditional logistic regression was applied to evaluate the association of the indel polymorphism with CSCC risk after adjusted for age, tobacco consumption, menopausal status and tumor stage. The normalized expression fold change of GAS5 in fresh tissue samples was compared with Student’s t-test. Logistic regression was used to carry out the stratified analysis on set, age, tobacco consumption, menopausal status between the rs531564 polymorphism and clinicopathological characteristics of CSCC, after adjusted for age, tobacco consumption, menopausal status and tumor stage. One-way ANNOVA was used to analyze the difference in luciferase reporter gene expression. The current statistical analysis was implemented on SPSS18.0. P<0.05 was seen as statistically significant.

Full table
Results
Clinicopathological features of enrolled subjects
Clinicopathological features are exhibited in Table 2. In discovery set, distribution of age indicates none marked difference (P=0.37), demonstrating that the current study is well matched in age. Other confounders such as tobacco consumption, menopausal status and tumor stage are also listed in Table 2. Distribution of these confounders shows none marked difference (P>0.05), except for tumor stage. The tendency of validation set is identical with that of discovery set.
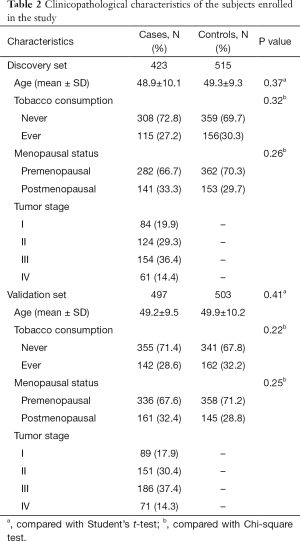
Full table
Positive relationship between rs145204276 and CSCC risk
Genotypic and allelic distribution of the indel polymorphism in discovery set are similar with the validation set, and genotypic distribution in controls of discovery set and validation set is in Hardy-Weinberg equilibrium (P=0.17 and P=0.11 respectively, data not shown). Thus, we furtherly performed pooled analysis for statistic power enlargement. In the pooled analysis, we applied four genetic models for detailed analysis. As it is revealed in Table 1, genotypic distribution in pooled controls still complies with Hardy-Weinberg equilibrium (P=0.13). When taking ins/ins genotype as reference in codominant model, ins/del genotype confers a slightly increased risk for CSCC (OR =1.27, 95% CI: 1.04–1.52, P=0.02), the homozygous del/del genotype obviously shows an increased risk for CSCC (OR =2.24, 95% CI: 1.60–2.97, P<0.00001), linear trend test confirms that the polymorphism and CSCC risk has linear correlation (Ptrend<0.00001). In recessive model, the homozygous del/del genotype shows an increased risk for CSCC (OR =1.98, 95% CI: 1.42–2.66, P<0.00001). The similar trend was also observed in dominant model. Data from additive model demonstrates that the del allele upregulates the CSCC risk (OR =1.42, 95% CI: 1.21–1.55, P<0.00001), indicating that the del allele possibly influences CSCC risk in dose-dependent manner. Although the highest value of OR is observed in codominant model, the ins/del genotype conferring OR value is too close to 1, while the second highest OR value from recessive model is away from 1. Thus, we took recessive model as our further stratified analysis model.
The del allele attenuates GAS5 expression
GAS5 expression among three genotype groups in CSCC tissues was observed to check whether the del allele influenced CSCC risk through modulation of adjacent gene GAS5. All 68 CSCC tissues were genotyped as 26 cases for ins/ins, 33 cases for ins/del and 9 cases for del/del. As it is revealed in Figure 1, in comparison with ins/ins genotyped cases, del/del cases show obvious decrease (P<0.01), and ins/del cases also display statistical difference (P<0.05). Thus, we have found the del allele could attenuate GAS5 expression and further modulate CSCC risk.

In vivo analysis has revealed the del allele could attenuate GAS5 expression, and in vitro analysis by transient transfection of dual luciferase reporter assay system has found that the luciferase activity of cells co-transfected with pGL3-WT or pGL3-MT is much lower than counterparts with empty pGL3-basic vector (P<0.01) (see Figure 2). The cells with pGL3-MT is more salient than cells with pGL3-WT in transcription activity comparison (P<0.05). Compared with Figure 1, the del/del genotype drives less expression fold change in vitro than in vivo.

Stratification analysis of rs145204276 and CSCC risk with potential confounders
We have found the positive correlation between rs145204276 and CSCC risk, and the influence is confirmed by in vitro and in vivo analysis, but its role in clinicopathological characters is not clear. Therefore, stratified analysis with potential confounders was performed in Table 3. The del/del genotype betrays an increased risk for CSCC in both set (both are P<0.01), positive correlation of rs145204276 and CSCC risk is prominent in senior age (>49 years) (P<0.00001), postmenopausal status (P<0.00001) and tobacco consumer (P<0.00001). The association of rs145204276 and tumor stage was also assessed in cases with tumor stages in Table 4. When taking tumor stage I cases as reference, salient association is found only in tumor stage IV (P=0.001).
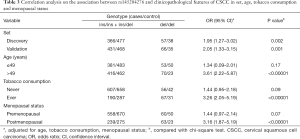
Full table
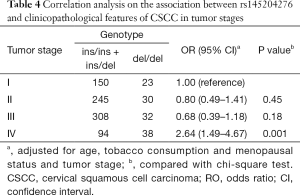
Full table
Discussion
It is the first epidemiological report that the indel polymorphism rs145204276 within lncGAS5 is correlated with CSCC risk, and the polymorphism could modulate GAS5 expression to affect CSCC risk. GAS5 was formerly known as a tumor repressor gene, but recent investigation found it acted as proto-oncogenic role in hepatocellular carcinoma. Based on the evidence of present investigation, GAS5 is regarded as a tumor suppressor in CSCC pathogenesis, this finding is in accordance with previous reports.
Data in Table 2 shows that risk factors such as age, tobacco consumption and menopausal status have no statistical difference in cases and controls. Former reports have revealed that HPV infection is a main risk factor for CSCC (14,15), and vaccine is the effective method to prevent CSCC (16). However, developing regions especially rural regions rarely have access to this relatively expensive prevention method. We collected the clinicopathological characters that were easy to obtain, thus incomplete data on HPV infection status was not collected in the current study.
In the present research, we performed two-stage case-control study to evaluate the association between rs145204276 and CSCC susceptibility. We designed discovery set and validation set to carry out the association investigation (data not shown). The discovery set was used to find out whether it had the association, and the validation set was applied to confirm our finding. The trends are similar in both sets, thus we put it into pooled analysis. The pooled analysis is listed in Table 1, and we have found that the trend is similar with discovery set and validation set (data not shown). We have got highest OR value for del/del genotype in codominant model, and have found the ins/del genotype confers a slightly increased risk for CSCC (OR =1.27). The second highest OR value is observed in recessive model, thus, we chose recessive model for further stratified analysis. We found the polymorphism rs145204276 could upregulate the CSCC risk, but the OR values are not prominent, thus the further stratified analysis was implemented to find the possibly stronger correlations.
Further stratified analysis in Table 3 shows that subjects with senior age (>49 years), in postmenopausal status and tobacco consumers confer stronger correlation with rs145204276 and CSCC risk. These risk factors have been demonstrated to be associated with CSCC risk in previous reports (17-19). The OR values in Table 3 are much higher than in Table 3, indicating there is a possible interaction between the polymorphism and risk factors, and the work is to be done in our subsequent research. The correlation analysis of tumor stage in CSCC cases has revealed that the polymorphism is associated with tumor stage IV based on the current data in Table 4. However, the enrolled cases of our current investigation are mainly distributed in tumor stage II and III and it would probably influence our analysis.
The possible genotype-phenotype correlation is confirmed in Figure 1. The GAS5 expression decreases while the del allele increases in fresh CSCC tissues, and the del allele could promote CSCC risk. Therefore, it has been inferred that decreased GAS5 expression promotes CSCC risk, and it has been in accordance with previous report (9). However, larger samples investigation is yet to be done for more clear knowledge on the anti-cancer role of GAS5.
The effect of del allele was further evaluated by in vitro assay. As shown in Figure 2, del allele could down-regulate promoter activity, it reverses the trend shown in HCC model (11). It has been postulated that the del allele could disrupt the motif that can bind certain transcription factors, but this hypothesis needs further validation from functional assays. Furthermore, the expression fold change of del/del genotype in vitro and in vivo is not equal, and this difference may be due to individual difference for gene expression not stable as cell lines. The in vitro analysis was implemented in three cell lines, whether other cell lines would show the similar trend is still to be validated.
In addition, the present investigation is an interesting finding, and the indel polymorphism rs145204276 within GAS5 would be a potential screening marker for CSCC diagnosis. However, much work such as candidate screening marker assessment and the efficacy compared with popular markers in CSCC diagnosis is to be done in the future.
The current investigation has several limitations. For example, the association is found only in Chinese Han ethnicity, population investigation from other ethnicities would facilitate the understanding of this functional polymorphism in disease. Another point we need focus on is that whether this polymorphism has the similar function in other cancers such as gastric cancer is yet to be elucidated.
Conclusions
In a word, in the present study we firstly report that the functional indel polymorphism rs145204276 could modulate CSCC risk in Chinese Han populations. GAS5 is involved in CSCC pathogenesis and its tumor suppressor role in CSCC is to be further confirmed.
Acknowledgments
We were grateful for the language editing help from Jude Juventus Aweya from National University of Singapore.
Funding: The present study was funded by Natural Science Foundation of China (grant number 81502428), Natural Science Foundation of Jiangsu Province (grant number BK20140222, 15KJB310024, BK20150220), and scientific research fund for talents of Xuzhou Medical University (grant number D2015018, D2015019).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.03.50). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committee of the Affiliated Hospital of Xuzhou Medical University and Shehong Hospital of Traditional Chinese Medicine (No. 2010123004) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wakeham K, Kavanagh K. The burden of HPV-associated anogenital cancers. Curr Oncol Rep 2014;16:402. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Pate Capps N, Stewart A, Burns C. The interplay between secondhand cigarette smoke, genetics, and cervical cancer: a review of the literature. Biol Res Nurs 2009;10:392-9. [Crossref] [PubMed]
- Chen D, Juko-Pecirep I, Hammer J, et al. Genome-wide association study of susceptibility loci for cervical cancer. J Natl Cancer Inst 2013;105:624-33. [Crossref] [PubMed]
- Chen D, Gyllensten U. Lessons and implications from association studies and post-GWAS analyses of cervical cancer. Trends Genet 2015;31:41-54. [Crossref] [PubMed]
- Nagano T, Fraser P. No-nonsense functions for long noncoding RNAs. Cell 2011;145:178-81. [Crossref] [PubMed]
- Peng L, Yuan X, Jiang B, et al. LncRNAs: key players and novel insights into cervical cancer. Tumour Biol 2016;37:2779-88. [Crossref] [PubMed]
- Ma C, Shi X, Zhu Q, et al. The growth arrest-specific transcript 5 (GAS5): a pivotal tumor suppressor long noncoding RNA in human cancers. Tumour Biol 2016;37:1437-44. [Crossref] [PubMed]
- Cao S, Liu W, Li F, et al. Decreased expression of lncRNA GAS5 predicts a poor prognosis in cervical cancer. Int J Clin Exp Pathol 2014;7:6776-83. [PubMed]
- Chang L, Li C, Lan T, et al. Decreased expression of long non-coding RNA GAS5 indicates a poor prognosis and promotes cell proliferation and invasion in hepatocellular carcinoma by regulating vimentin. Mol Med Rep 2016;13:1541-50. [PubMed]
- Tao R, Hu S, Wang S, et al. Association between indel polymorphism in the promoter region of lncRNA GAS5 and the risk of hepatocellular carcinoma. Carcinogenesis 2015;36:1136-43. [Crossref] [PubMed]
- Allen RC, Graves G, Budowle B. Polymerase chain reaction amplification products separated on rehydratable polyacrylamide gels and stained with silver. Biotechniques 1989;7:736-44. [PubMed]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402-8. [Crossref] [PubMed]
- Ibeanu OA. Molecular pathogenesis of cervical cancer. Cancer Biol Ther 2011;11:295-306. [Crossref] [PubMed]
- Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet 2007;370:890-907. [Crossref] [PubMed]
- Markman M. Human papillomavirus vaccines to prevent cervical cancer. Lancet 2007;369:1837-9. [Crossref] [PubMed]
- Darlin L, Borgfeldt C, Widén E, et al. Elderly women above screening age diagnosed with cervical cancer have a worse prognosis. Anticancer Res 2014;34:5147-51. [PubMed]
- Sawaya G, Sung H, Kearney K, et al. Invasive cervical cancer in members of a prepaid health plan: the effect of menopausal status on disease characteristics. J Low Genit Tract Dis 1999;3:41. [Crossref] [PubMed]
- Brinton LA, Schairer C, Haenszel W, et al. Cigarette smoking and invasive cervical cancer. JAMA 1986;255:3265-9. [Crossref] [PubMed]


