
DKK4 is important in Snail1-induced chemoresistance to fluorouracil in colorectal cancer
Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors worldwide (1). In clinical, the prognostic assessment of CRC is traditionally based on the TNM stage. The advanced the TNM stage is correlated with the poorer prognosis. In addition to the TNM stage, the level of CEA, tumor histopathological grade and other factors like KRAS, PIK3A mutations all influence the prognosis. Currently, molecular classification of CRC is a promising prognostic system which requires a deeper understanding of the molecular mechanism of tumor initiation, progression, invasion and resistance to chemotherapy (2,3).
5-fluorouracil (5-FU) has been the most frequently used chemotherapy for advanced CRC for the last 40 years. Despite the widespread use of the 5-FU, resistance to 5-FU-based chemotherapy remains a major problem in the treatment of CRC (4). Although the prognosis for patients with advanced CRC has improved because of the development of novel therapeutic strategies (5), unmasking the underlying mechanism of CRC chemoresistance could lead to novel therapeutic strategies.
Snail1, a Zinc-finger transcription factor family member, is one of the most important EMT related transcriptional regulators (6,7). Snail1 is overexpressed in many malignant cancers, including CRC (8). Snail1 is overexpressed in CRC, and it is inversely correlated with the E-cadherin level in CRC (8). Snail1 has also been demonstrated to have crucial roles in CRC by regulating prostaglandin E2 and vitamin D (9-11). Notably, Snail1 can suppress the expression of Vitamin D receptor (VDR) by binding to the promoter region of exon 1a of the human VDR gene (12,13). Therefore, a high level of Snail1 may be responsible for the failure of Vitamin D analogue therapy in CRC patients (10,14). The overexpression of Snail1 can promote EMT, cancer stem cell growth and metastasis, and cancer stem cells are correlated with chemoresistance in many cancers. However, the correlation between Snail1 and chemoresistance in CRC as well as the underlying mechanism of Snail1-induced chemoresistance remain unknown.
The Dickkopf (DKK) family, which consists of 4 secretory glycoproteins, namely DKK1, DKK2, DKK3, and DKK4, can suppress canonical Wnt signaling by binding to low-density lipoprotein receptor-related protein-5 (LRP5) (15,16).There is a hyper- or hypo-expression of DKK4 in many different malignant diseases (17-19), and DKK4 is reported to be correlated with chemoresistance in CRC (20,21). Because few compounds have been developed to target Snail1, uncovering Snail-regulated-genes that can be targeted by drugs could contribute to the development of new therapeutic strategies against Snail1-mediated chemoresistance. Therefore, the aim of this study was to analyze the co-expression patterns of Snail1 and DKK4 in CRC samples and their relationship to chemoresistance. Further, we tested the effects of Snail1 and DKK4 in CRC cell lines. Here, we showed that the high expression level of Snail1 was correlated with poor prognosis and chemoresistance in CRC. The overexpression of Snail1 in CRC cell lines could result in resistance to 5-FU treatment by upregulating the DKK4 level. The DKK4 expression level was correlated to Snail1 expression in CRC. Therefore, our results indicated that a novel Snail1/DKK4 regulation axis in CRC which is involved in the chemoresistance of CRC.
Methods
Patients and tissue samples
Patients with histologically confirmed CRC who received chemotherapy after curative resection in Xinhua Hospital were included in this study. Patients who underwent neoadjuvant treatment or R1 resection were excluded from the analysis. Formalin-fixed, paraffin-embedded tumor blocks were collected. Patients with local recurrence or distant metastasis <6 months after chemotherapy were classified as having a chemoresistance response, and those with local recurrence or distant metastasis for >6 months were classified as having a chemosensitive response (22-24).
Immunohistochemistry staining and scoring
Immunohistochemical detection of Snail1 and DKK4 was performed as previously described (25). In short, the paraffin sections were treated according to a standard protocol. After incubating with the anti-Snail1 antibody and anti-DKK4 antibody overnight, sections were washed 3 times with PBS and incubated with HRP-conjugated secondary antibody (GK500710; Gene Company Ltd.) for 30 min at RT. Then, staining was developed with DAB solution for 10 seconds. The sections were counterstained with 0.1% hematoxylin and then sealed with coverslips.
IHC scoring of DKK4 was previously described (26,27). DKK4 staining in tissues was scored by the staining intensity (0-lack of staining, 1-mild staining, 2-moderate staining, and 3-strong staining), and the score was multiplied by of the percentage of staining {[1] <25%, [2] 25–50%, [3] 51–75%, and [4] >75%}, which ranged from 0-12. When the multiplication score was >6, the DKK4 expression was considered high. The methods for scoring Snail1 are described in a previous study (27). Two histopathologists were blindly assigned to review the slides and score the staining.
Cell culture
The human CRC cell lines, SW480, HCT116 and LoVo, were purchased from American Type Culture Collection(ATCC).SW480, HCT116 and LoVo were cultured in Dulbecco’s modified Eagle's medium (Hyclone) with 10% fetal bovine serum (Gibco) and 1% penicillin & streptomycin (Invitrogen).
RNAi transfection
The siRNA of Snail1 and DKK4 were purchased from Genepharma, and the sequences are listed below. In brief, si-DKK4, si-Snail1 and negative control siRNA were transfected into CRC cell lines using Lipofectamine 2000 according to the manufacturer’s protocol (Thermo Fisher Scientific).
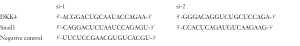
Full table
RNA extraction and quantitative real-time PCR
Total RNA was extracted using Trizol (TaKaRa).Complementary DNA was synthesized with PrimeScript RT-PCR Kit (TaKaRa). Quantitative RT-PCR was performed using SYBR Premix ExTaq (TaKaRa) on an ABI 7,500 real time PCR system (Applied Biosystems). The primer sequences for mRNA qRT-PCR are indicated below. The procedures for qPCR were as follows: initial denaturation at 95 °C for 1min, followed by 40 cycles of heating to 95 °C for 5 s and then at 60 °C for 34 s. The relative gene expression levels were calculated using the 2–ΔΔCt analysis method.
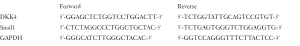
Full table
Vector construction, retrovirus production and transduction
The full length of the Snail1 CDS region was inserted in the pBABE-puro vector by using the BamHI/EcoRI sites. In terms of the virus package, GAG and VSVG were co-transfected into 293T cells with pBABE-puro or pBABE-Snail1-puro by using the Polyethylenimine linear (PEI, polysciences). Then, 48 to 72 hours later, virus particles were harvested for concentration. Cell lines were infected with retrovirus overnight with 1 µg/mL polybrene (Sigma) before fresh medium was added. Cells were then selected for 1 week using puromycin.
Cell viability assay
For this, 5-flurouracil (Sigma-Aldrich) was used in our study. SW480 and HCT116 cells were seeded in 96-well plates (1.5×104 for SW480 and 1×104 for HCT116).Then, 24 hrs later, the cells were treated with 5-FU. After 48 hrs, 20 µL of CCK8 (Dojindo) was added to each well, and the plates were incubated at 37 °C for one and a half hours. Then, the absorbance was measured with a spectrophotometer at 450 nm.
Statistics
Statistical analyses were applied using the SPSS 19.0 software. Student’s t-test was used for statistical analysis. Survival curves were plotted using the Kaplan-Meier method and compared with the log-rank test. The Pearson χ2 test was used to assess the correlation between Snail1 expression and clinical parameters. Univariate and multi-variate analyses were obtained using the Cox proportional hazards regression model. A two-sided P<0.05 was considered statistically significant.
Results
Increased Snail1 expression is correlated with chemoresistance and poor prognosis in CRC
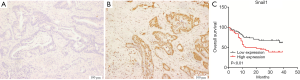
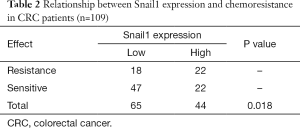
Snail1 regulates cancer stem cells in human colorectal carcinoma cells (28). Stemness can lead to cancer cell resistance to the chemotherapy. We performed an immunohistochemical analysis of Snail1 in 109 paraffin-embedded CRC tissue samples (Figure 1A,B). No significant difference between the Snail1 expression level and clinicopathological features, except for gender, was observed in our cohort (Table 1). Interestingly, a high expression level of Snail1 was associated with a poor prognosis in the CRC (Figure 1C).We observed that the resistance to 5-FU-based chemotherapy was highly correlated with Snail1 (P=0.018, Table 2).As a result, the overexpression of Snail1 could be a potential indicator of poor prognosis and chemoresistance in CRC. Additionally, the univariate analyses indicated that Snail1 expression, lymph node metastasis and distant metastasis were correlated with the overall survival (Table 3), whereas the multi-variate analyses showed that Snail1 expression and distant metastasis were independent prognostic factors for overall survival (Table 4).

Full table
Full table
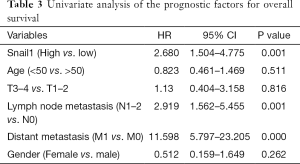
Full table
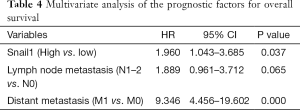
Full table
Overexpression of Snail1 in CRC cell lines leads to resistance to 5-FU treatment
To investigate the role of Snail1 in chemoresistance in CRC, Snail1 was cloned into the pBABE vector, which was utilized to generate stable cells overexpressing Snail1 in HCT116 and SW480 cells. We confirmed the overexpression of Snail1 in both cell lines using qPCR (Figure 2A). Then, the cell stably expressing Snail1 was treated with 5-FU, and the number of surviving cells was measured by CCK8. After 48hrs of 5-FU treatment, SW480-pBABE-Snail1 and HCT116-pBABE-Snail1 cells showed a significant increase in cell survival compared to the SW480-pBABE and HCT116-pBABE vector control cells, respectively (Figure 2B,C). These results were consistent with the clinical observation of an association between Snail1 overexpression and chemoresistance in CRC.

Snail1 promotes chemoresistance by upregulating the DKK4 level in CRC
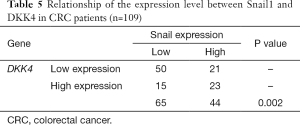
Next, we explored the underlying mechanism of Snail1-induced chemoresistance in CRC. DKK4 is reported to be involved in the chemoresistance induced by the hypermethylation of TFAP2E gene in CRC. Intriguingly, we found that knockdown of Snail1 in LoVo cells downregulated DKK4 mRNA level (Figure 3A) while overexpression of Snail1 had the opposite effect in both HCT116 and SW480 cells (Figure 3B). To further determine the function of DKK4 in Snail1-induced chemoresistance, two different siRNAs targeting DKK4 were used to knockdown the DKK4 level in Snail1-overexpressing cell lines. Knockdown of DKK4 was confirmed by qPCR (Figure 3C). Cells transfected with siRNA were seeded in 96-well plates and treated with different concentrations of 5-FU, and the cell viability was measured by CCK8. Consistent with our predication, knockdown of DKK4 in Snail1-overexpressing cell lines led to chemo-sensitivity in these cell lines (Figure 3D,E). Furthermore, a significant correlation between DKK4 and Snail1 was observed in our cohort (Figure 3F,Table 5). Taken together, our results indicated that DKK4, a vital factor in chemoresistance (29,30), is involved in Snail1-induced chemoresistance in CRC.
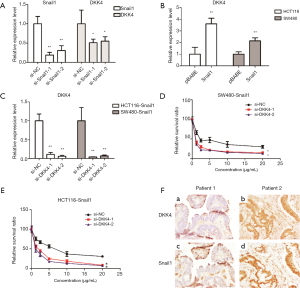
Full table
Discussion
Snail1,a member of Snail superfamily of transcription factors, has been implicated in many processes related to EMT and the inheritance of stem cell properties (31-33).Snail1 is regulated by LOXL2, SMAD, and HMGA2 by directly binding to the Snail1 promoter (34-36). Snail1 exerts a great role in the development of human tumors, including CRC, which is one of the most aggressive cancers (37). A high level of Snail1 in CRC was correlated with advanced stage and chemoresistance. In our cohort, we found that a high level of Snail1 in CRC patients results in resistance to 5-FU-based chemotherapy. In the CRC cell line, we also observed chemoresistance that was induced by the overexpression of Snail1. These results provide a possibility for Snail1 to act as a predictor of a response to chemotherapy.
Dysregulation of DKK4 has been frequently observed in several human malignancies, including hepatocellular carcinoma, pancreatic cancer, renal cell carcinoma and CRC (18,19). In CRC, DKK4 is involved in the chemoresistance. Induction of DKK4 expression results in the resistance to the 5-FU treatment. Transcription factor TFAP2E inhibits DKK4 expression in CRC cells and hypermethylation of TFAP2E gene is correlated with chemoresistance as well as increased expression of DKK4 in the CRC (20,21,38). Here, our results demonstrate that the up-regulation of DKK4 by Snail1 in CRC led to chemoresistance in the CRC cell lines. Furthermore, a high level of DKK4 was not only associated with poor response to chemotherapy, but DKK4 level is positively correlated with Snail1 in our cohort. In CRC cell lines, knockdown of DKK4 reversed the chemoresistance induced by Snail1.These findings suggested that DKK4 may be a potential downstream target of Snail1 and has an important role in Snail1-inducedchemoresistance. Snail1 is a transcriptional co-repressor (31) but can also function as a transcriptional co-activator (39). Thus, Snail1 can promote the transcription of DKK4 directly or indirectly. The underlying mechanism need to be explored in the future.
In summary, together with previous results, this study showed that Snail1 and DKK4 were frequently up-regulated in CRC patients and CRC cell lines. Additionally, the expression levels of Snail1 and DKK4 were associated with an advanced stage and chemoresistance in our cohort. In CRC cell lines, the overexpression of Snail1 not only resulted in resistance to 5-FU treatment, but it also increased the DKK4 expression level. In addition, knockdown of DKK4 in stably expressingSnail1 cell lines decreased the resistance to 5-FU that was induced by Snail1, which suggests DKK4 plays an important role in Snail1-induced chemoresistance. Because Snail1 is difficult to target by using the currently available drugs, targeting DKK4 in patients who are resistant to 5-FU may be a promising therapeutic strategy.
Acknowledgments
Funding: The study was supported by the National Natural Science Foundation of China (Grant No. 81372636, No. 81570474, No. 81502020) and the Fund from Shanghai Jiaotong University, School of Medicine (4XJ10030) and Xinhua Hospital (13YJ06).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.03.18). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Xinhua Hospital, Shanghai Jiaotong University School of Medicine (Approval No. XHEC-D-2011-047) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Trinh A, Trumpi K, De Sousa E, Melo F, et al. Practical and Robust Identification of Molecular Subtypes in Colorectal Cancer by Immunohistochemistry. Clin Cancer Res 2017;23:387-98. [Crossref] [PubMed]
- De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol 2010;11:753-62. [Crossref] [PubMed]
- Dy GK, Hobday TJ, Nelson G, et al. Long-term survivors of metastatic colorectal cancer treated with systemic chemotherapy alone: a North Central Cancer Treatment Group review of 3811 patients, N0144. Clin Colorectal Cancer 2009;8:88-93. [Crossref]
- Bardelli A, Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol 2010;28:1254-61. [Crossref] [PubMed]
- Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 2007;7:415-28. [Crossref] [PubMed]
- Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol Biol Cell 2008;19:4875-87. [Crossref] [PubMed]
- Fan F, Samuel S, Evans KW, et al. Overexpression of snail induces epithelial-mesenchymal transition and a cancer stem cell-like phenotype in human colorectal cancer cells. Cancer Med 2012;1:5-16. [Crossref] [PubMed]
- Backlund MG, Mann JR, Holla VR, et al. Repression of 15-hydroxyprostaglandin dehydrogenase involves histone deacetylase 2 and snail in colorectal cancer. Cancer Res 2008;68:9331-7. [Crossref] [PubMed]
- Pálmer HG, Larriba MJ, García JM, et al. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat Med 2004;10:917-9. [Crossref] [PubMed]
- Peña C, García JM, Silva J, et al. E-cadherin and vitamin D receptor regulation by SNAIL and ZEB1 in colon cancer: clinicopathological correlations. Hum Mol Genet 2005;14:3361-70. [Crossref] [PubMed]
- Pálmer HG, González-Sancho JM, Espada J, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol 2001;154:369-87. [Crossref] [PubMed]
- Cross HS, Bajna E, Bises G, et al. Vitamin D receptor and cytokeratin expression may be progression indicators in human colon cancer. Anticancer Res 1996;16:2333-7. [PubMed]
- Larriba MJ, Muñoz A. SNAIL vs vitamin D receptor expression in colon cancer: therapeutics implications. Br J Cancer 2005;92:985-9. [Crossref] [PubMed]
- Krupnik VE, Sharp JD, Jiang C, et al. Functional and structural diversity of the human Dickkopf gene family. Gene 1999;238:301-13. [Crossref] [PubMed]
- Chen L, Li M, Li Q, et al. DKK1 promotes hepatocellular carcinoma cell migration and invasion through β-catenin/MMP7 signaling pathway. Mol Cancer 2013;12:157. [Crossref] [PubMed]
- Chen HJ, Sun J, Huang Z, et al. Comprehensive models of human primary and metastatic colorectal tumors in immunodeficient and immunocompetent mice by chemokine targeting. Nat Biotechnol 2015;33:656-60. [Crossref] [PubMed]
- Ouyang Y, Pan J, Tai Q, et al. Transcriptomic changes associated with DKK4 overexpression in pancreatic cancer cells detected by RNA-Seq. Tumour Biol 2016;37:10827-38. [Crossref] [PubMed]
- Chouhan S, Singh S, Athavale D, et al. Glucose induced activation of canonical Wnt signaling pathway in hepatocellular carcinoma is regulated by DKK4. Sci Rep 2016;6:27558. [Crossref] [PubMed]
- Giovannetti E, Codacci-Pisanelli G, Peters GJ. TFAP2E-DKK4 and chemoresistance in colorectal cancer. N Engl J Med 2012;366:966-author reply 966. [Crossref] [PubMed]
- Ebert MP, Tänzer M, Balluff B, et al. TFAP2E-DKK4 and chemoresistance in colorectal cancer. N Engl J Med 2012;366:44-53. [Crossref] [PubMed]
- Yao L, Zhang Y, Chen K, et al. Discovery of IL-18 as a novel secreted protein contributing to doxorubicin resistance by comparative secretome analysis of MCF-7 and MCF-7/Dox. PLoS One 2011;6:e24684 [Crossref] [PubMed]
- Cheng WF, Huang CY, Chang MC, et al. High mesothelin correlates with chemoresistance and poor survival in epithelial ovarian carcinoma. Br J Cancer 2009;100:1144-53. [Crossref] [PubMed]
- Wu T, Wang Z, Liu Y, et al. Interleukin 22 protects colorectal cancer cells from chemotherapy by activating the STAT3 pathway and inducing autocrine expression of interleukin 8. Clin Immunol 2014;154:116-26. [Crossref] [PubMed]
- Tang W, Zhu Y, Gao J, et al. MicroRNA-29a promotes colorectal cancer metastasis by regulating matrix metalloproteinase 2 and E-cadherin via KLF4. Br J Cancer 2014;110:450-8. [Crossref] [PubMed]
- Liu Y, Wang G, Yang Y, et al. Increased TEAD4 expression and nuclear localization in colorectal cancer promote epithelial-mesenchymal transition and metastasis in a YAP-independent manner. Oncogene 2016;35:2789-800. [Crossref] [PubMed]
- Schwock J, Bradley G, Ho JC, et al. SNAI1 expression and the mesenchymal phenotype: an immunohistochemical study performed on 46 cases of oral squamous cell carcinoma. BMC Clin Pathol 2010;10:1. [Crossref] [PubMed]
- Hwang WL, Yang MH, Tsai ML, et al. SNAIL regulates interleukin-8 expression, stem cell-like activity, and tumorigenicity of human colorectal carcinoma cells. Gastroenterology 2011;141:279-91, 291.e1-5.
- Xi Y, Formentini A, Nakajima G, et al. Validation of biomarkers associated with 5-fluorouracil and thymidylate synthase in colorectal cancer. Oncol Rep 2008;19:257-62. [PubMed]
- Xi Y, Nakajima G, Schmitz JC, et al. Multi-level gene expression profiles affected by thymidylate synthase and 5-fluorouracil in colon cancer. BMC Genomics 2006;7:68. [Crossref] [PubMed]
- Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol 2002;3:155-66. [Crossref] [PubMed]
- Wang H, Zhang G, Zhang H, et al. Acquisition of epithelial-mesenchymal transition phenotype and cancer stem cell-like properties in cisplatin-resistant lung cancer cells through AKT/β-catenin/Snail signaling pathway. Eur J Pharmacol 2014;723:156-66. [Crossref] [PubMed]
- Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 2005;132:3151-61. [Crossref] [PubMed]
- Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, et al. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J 2005;24:3446-58. [Crossref] [PubMed]
- Thuault S, Tan EJ, Peinado H, et al. HMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-to-mesenchymal transition. J Biol Chem 2008;283:33437-46. [Crossref] [PubMed]
- Brandl M, Seidler B, Haller F, et al. IKK(α) controls canonical TGF(ß)-SMAD signaling to regulate genes expressing SNAIL and SLUG during EMT in panc1 cells. J Cell Sci 2010;123:4231-9. [Crossref] [PubMed]
- Cunningham D, Atkin W, Lenz HJ, et al. Colorectal cancer. Lancet 2010;375:1030-47. [Crossref] [PubMed]
- Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med 2009;361:2449-60. [Crossref] [PubMed]
- Hsu DS, Wang HJ, Tai SK, et al. Acetylation of snail modulates the cytokinome of cancer cells to enhance the recruitment of macrophages. Cancer Cell 2014;26:534-48. [Crossref] [PubMed]


