
Survival benefit of neoadjuvant radiation therapy in patients with carcinoma of gastroesophageal junction and distal esophagus? A population-based study
Introduction
The incidence of carcinoma of gastroesophageal junction (GEJ) and lower third esophageal cancer (EC) is rising steadily in both Western and Eastern countries (1). Advancements in multimodality therapy contribute to the survival of locally advanced diseases, although the prognosis is still poor (2). Meanwhile, there is still controversy in multidisciplinary treatment, such as the different combinations of surgery, radiation and chemotherapy, treatment sequence, etc. Also, some of the existing evidence relevant to this entity of cancer is based on EC or gastric cancer (GC), including not only cardiac gastric adenocarcinoma (GAC), esophageal squamous cell carcinoma (ESCC), and esophageal adenocarcinoma (EAC) of distal esophagus (DE) but also non-cardiac GC, cervical and thoracic EC, which contributes to the inaccuracy and uncertainty (3). The definition of GEJ cancer varies in documents and is reported differently even by reputable experts and societies in the world (4).
Siewert et al. proposed a definition and an anatomic classification system in the 1980s, which was clinical and based on barium esophagram, endoscopic examination of the esophagogastric junction, computed tomography, and observations during operations (Table S1) (5,6). Siewert defined GEJ as the “upper end of the typical longitudinal fold of the gastric mucosa.” This was an anatomic or endoscopic definition that could guide decisions made preoperatively, but allowing for intraoperative findings to be included for guidance (7). American Joint Committee on Cancer (AJCC) adopted an objective and easily applied staging system different from the existing Siewert classification based on postoperative pathology in 2010 (8). AJCC considers GEJ lesions to be a subtype of EC and also has harmonized important aspects of the staging of esophageal and GC (9). In the new staging system, GEJ tumors are defined as EC (rather than GC) the center of which is in the distal thoracic esophagus, GEJ, or in the proximal 5 cm of the stomach (cardia) that involves the GEJ or distal thoracic esophagus (10). The staging system also includes altered nodal and T staging, separation of adenocarcinoma and squamous cell carcinoma, and incorporation of histological grade (11).
Data support the concept that adenocarcinoma of GEJ and DE might be an entity of disease and treatment could be guided by the same principles, though the validity is unproven at this time (4). To date, the population-based data about the use and effectiveness of surgery and radiation therapy for carcinoma of GEJ and DE is very limited (12). Prospective clinical trials focused on this entity of disease is lacking, and in published trials, patients with carcinoma of GEJ and DE were included as part of subjects studied, which also comprised patients with GC or thoracic EC. In spite of existing data, there is still a lot of controversy about the treatment of this entity of disease (13).
In this study Carcinoma of abdominal esophagus was included and considered as “carcinoma of gastroesophageal junction and distal esophagus”. We analyzed the Surveillance Epidemiology and End Results (SEER) data base to evaluate outcomes of esophageal cancer-specific survival (CSS) and overall survival (OS) of patients with carcinoma of GEJ and DE treated by different modalities.
Methods
Study population and data source
The SEER data between January 1973 and December 2012 [“Incidence − SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2014 Sub (1973–2012 varying)”] was chosen and the National Cancer Institute’s SEER*Stat software (Version 8.2.1) was used for the identification of patients. The inclusion criteria contained: (I) primary EC (C15.0–C15.9) with a confirmed diagnosis of microscopically; (II) Anatomic site located in the regional abdominal esophagus; (III) total histology based on the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3). And the exclusion criteria contained: (I) unknown age, sex, race; (II) with a radiotherapy (RT) status of “radiation both before and after surgery”, “intraoperative radiation therapy”, “intraoperative radiation with other radiation given before or after surgery”, “surgery both before and after radiation (for cases diagnosed 1/1/2012 and later)”, or “sequence unknown, but both surgery and radiation were given”; (III) diagnosed solely on autopsy or death certificate. Survival data were extracted at 1-month intervals for a maximal follow-up of 60 months. This study is based on public data from the SEER database. The reference numbers that were obtained for the permission to access research data files was 10612-Nov2014. No human subjects or personal identifying information were used in this study. This study was approved by the Review Board of Nanjing Medical University, Nanjing, China.
Statistical analysis
The enrolled population was divided into five groups based on different treatment: patients who were treated with surgery alone (Surg group), with RT alone (RT group), with surgery following with RT (RT + Surg group), with surgery followed by RT (Surg + RT group) and patients who received no surgery or RT. Chi-square test was used to analyze the differences between categorical variables of these five groups. Survival curves were plotted by Kaplan-Meier method and log-rank test was used for comparison. Multivariate analysis with cox proportional hazards regression model was performed to examine the clinical factors’ association with survival respectively. Finally, stratified cox regression survival analysis was performed. All data were analyzed using SPSS Statistics software (version 19.0; IBM Corporation, USA). Statistical significance was defined as P value less than 0.05.
Results
A total of 516 patients with lower third (abdominal) EC, who fulfilled the inclusion and exclusion criteria during the period between 1973 and 2012 were identified from the SEER database. Of these, 135 of them received no surgery or RT, 139 and 164 had surgery and RT alone, respectively, 41 had RT before surgery and 37 had RT after surgery. The baseline characteristics of patients in these five treatment groups are shown in Table 1.
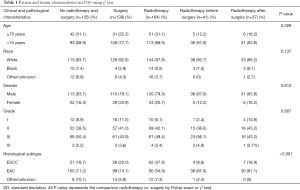
Full table
Factors including age, gender, race, histological type, surgery, radiation and cause-specific death classification were included in multivariable Cox regression analysis. We included these factors in this analysis as relevant data was available in SEER database, while other prognostic factors, such as TNM stage, were missing in most cases. In all the 516 patients, multivariable Cox regression analysis showed that factors independently predicted the OS of patients with carcinoma of GEJ and DE included gender [female vs. male: hazards ratio (HR) 0.667, 95% CI: 0.518–0.858, P=0.002], histological type (ESCC vs. EAC: HR 1.409, 95% CI: 1.109–1.790, P=0.005), surgery (no vs. yes: HR 2.837, 95% CI: 2.271–3.542, P<0.001), RT (no vs. yes: HR 1.608, 95% CI: 1.316–1.964, P<0.001) (Table 2).
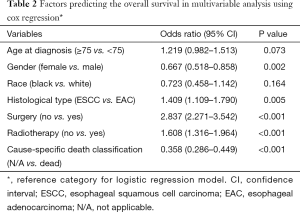
Full table
In the multivariable analyses of overall and CSS of patients carcinoma of GEJ and DE (Table 3), patients benefited from surgery or RT, regardless of histological subtype. For all the 516 patients with carcinoma of GEJ and DE, the most significant treatment modality was surgery combined with preoperative RT, which improved both OS (HR 0.182, 95% CI: 0.118–0.280, P<0.001) and CSS (HR 0.179, 95% CI: 0.108–0.295, P<0.001) remarkably. RT alone was inferior to surgery alone in both OS (HR 2.217, 95% CI: 1.680–2.817, P<0.001) and CSS (HR 2.215, 95% CI: 1.620–3.028, P<0.001). In patients with ESCC (n=126), patients receiving surgery combined with postoperative RT appeared to have a worse outcome than those receiving surgery alone [SurgRT vs. Surg: HR 3.422 (1.337–8.761), P=0.010] or those receiving surgery combined with preoperative RT [RTSurg vs. SurgRT: HR 0.158 (0.031–0.800), P=0.026].
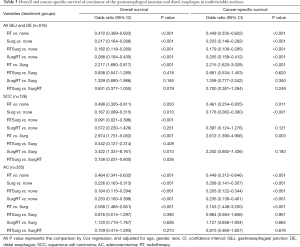
Full table
Discussion
In this study, 331 cases of EAC, 119 cases of ESCC as well as 32 cases of other types of EC were included. The survival of patients with SCC of GEJ and DE was significantly worse than that of patients with EAC. This result might be attributed to the different treatments utilized in these two groups of diseases. Moreover, it could not be excluded that the tumor characteristics including TNM staging varied between these two groups. However, the TNM staging data was lacking in most cases included, which prevented us from further study. In a study by Yoshikawa et al., in 431 patients with cancers at GEJ (381 EACs and 50 ESCCs), the 5-year OS rates were 60.4% (55.1–65.7%) in the EAC group and 52.3% (35.6–69.0%) in the ESCC group (14). And in a study of 123 patients with Siewert type II GEJ esophageal carcinoma, patients with ESCC presented with more advanced stages, although the 5-year OS rate did not show significant difference. However, the above two studies did not support our findings directly (15).
Cases of ESCC or EAC of GEJ and DE received surgery alone or RT had a survival advantage over those received either surgery alone or RT. It is possible that some of patients who were treated with either surgery or RT received chemotherapy alone, however, the chemotherapy data was not available. However, in both patients with ESCC and EAC of GEJ and DE, perioperative RT did not show additional benefits on OS or CSS, while compared with surgery alone. We also found that patients receiving surgery and postoperative radiation had a worse outcome than those receiving surgery alone. These results may be caused by the small number of patients treated with surgery combined with perioperative RT or the fact that the basic characteristics of tumor may differ significantly between the different groups. Existing meta-analyses suggested that preoperative radiation provided a survival benefit, the results were not significant (16,17). This result may partly explain that preoperative RT is not superior to surgery alone in our study.
In western countries, the majority of patients with have locally advanced disease at their presentation, and the outcome of patients treated by surgery alone is dismal with a 5-year survival rate of approximately 25% in multiple population-based registries, which has not significantly changed since several decades ago (18). The current treatment of carcinoma of GEJ and DE requires the inclusion of multidisciplinary management due to the advanced disease and the treatment-associated morbidities and early progression of disease. Optimal management is controversial and considerable variations in the treatment approaches exist. For adenocarcinoma of GEJ and DE, surgery with any level of lymph node dissection followed by adjuvant chemoradiation improved survival compared to surgery alone and is now accepted by some practitioners in the US (19). Similarly, preoperative chemotherapy (CT) improved outcome compared to surgery alone in patients with gastroesophageal adenocarcinomas and is widely applied in Europe and Australasia (20). For esophageal adenocarcinoma, neoadjuvant chemoradiation and neoadjuvant chemotherapy are widely used in the US and UK respectively. And for localized ESCC, patients benefit from chemoradiation, which could be used as a neoadjuvant or definitive therapy, with the latter one decreasing surgery-associated morbidity and mortality (20).
RT as a neoadjuvant strategy has been utilized for optimal locoregional tumor control. Preoperative chemoradiotherapy (CRT) provides several advantages. Firstly, the location of the primary cancer can be detected more precisely, which makes the planning of radiation fields more effective and accurate. Secondly, the preoperative therapy can allow enough time to observe high-risk patients for progression (21). Trials reported by Walsh et al. (22), Urba et al. (23), and Tepper et al. (24) demonstrated a benefit of CRT, whereas other randomized trials such as that reported by Burmeister et al. (25) did not confirm a benefit. Recently, the larger CRT for Esophageal Cancer Followed by Surgery Study (CROSS) trial has proved the effectiveness of neoadjuvant chemoradiation, in which 24% had GEJ tumors, and an additional 58% had lesions of DE (26,27).
Although CRT and CT are both adapted as neoadjuvant therapies, evidence suggesting that CRT is superior to CT has emerged in the last years. Stahl et al. conducted a phase III trial, which included 126 patients with adenocarcinoma in lower third of esophagus and cardia, compared neoadjuvant CT followed by surgery with neoadjuvant CRT followed by surgery (28). However, the postoperative mortality was higher in the CRT group than in the CT group (10.2% vs. 3.8%). Although the study was terminated prematurely and differences between two groups were not of statistical significance, results indicated a survival superiority for CRT as compared to CT in adenocarcinoma of GEJ.
Details for selected trails comparing neoadjuvant (chemo)radiotherapy with surgery alone for carcinoma of GEJ and DE are listed in Table 4 (22-30). A recent meta-analysis including 12 randomized EC trials Which compared neoadjuvant CRT to surgery alone (including CROSS) drawed a conclusion that CRT was significantly associated with improved OS (HR 0.78, 95% CI: 0.70–0.88; P<0.0001) (31). Survival benefit from neoadjuvant CRT was achieved in both ESCCs (HR 0.80; 95% CI 0.68–0.93; P=0.004) and EACs (HR 0.75; 95% CI: 0.59–0.95; P=0.02), dispelling the view that only ESCC benefit from CRT. In the same meta-analysis, although both neoadjuvant CRT and preoperative CT improved survival as compared to surgery alone, greater benefit was observed with CRT.
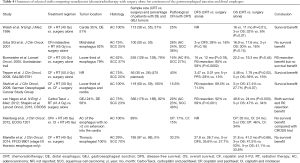
Full table
Results for selected studies comparing adjuvant (chemo)radiotherapy with surgery alone for carcinoma of GEJ and DE are summarized in Table 5 (32-34). The US intergroup study reported by MacDonald et al. (Southwest Oncology Group [SWOG] 9008/Intergroup [INT] 0116) (32,33) demonstrated significantly improved survival for patients with GC and adenocarcinoma of GEJ treated by postoperative chemoradiation compared with surgery alone. This trial enrolled patients after R0 resection with pathological stage from IB (T1N1M0 or T2N0M0) to IVM0 (T4NanyM0) according to the 1988 gastric staging system at that time. In this trial, 20% of patients had GEJ tumors, and this subgroup has not been separately analyzed. Only patients who had undergone an R0 resection were eligible, making it difficult to directly compare the results with those from trials of neoadjuvant therapies in which the pathological results were not restricted to R0 resection. Adjuvant treatment consisted of 5-FU plus leucovorin and 45 Gy of radiation. Median survival was improved from 27 to 36 months, but grade 3 and 4 chemoradiation toxicity was 41% and 32%, respectively. Three-year survival rate was improved from 41% to 50%, with survival remaining improved at least 10 years of follow-up (HR 1.32; 95% CI, 1.10–1.60; P=0.0046). These survival results suggest that this is an appropriate option for those patients who have had surgery without preoperative therapy, although the outcome for GEJ tumors alone has not been available.

Full table
Published trials showed promising results of pre- and post-operation RT for GEJ and DE tumors, however almost all the studies included predominantly esophageal or GC. Since GEJ tumors is considered more as EC rather than GC and can have similar management strategy, we overview the existing resulting from the big data of SEER and related published trails, aiming at showing the landscape of clinical research of this disease and providing some information for future prospective clinical trials.
However, there are a number of limitations in our study. The SEER database does not collect several important prognostic factors, such as coexisting morbidities, provider information, treatment-related complications, details on surgical procedure (open surgery or minimally invasive techniques), and disease recurrence. And for most patients, the SEER database does not provide the TNM stage information. Besides, the data about chemotherapy, which plays a potentially important role in the treatment of carcinomas of GEJ, were not available to us. Therefore, we were unable to adjust for these factors in our survival analysis. Since this is a non-randomized study, our results could be influenced by selection bias and confounders. Another weakness of our study is that not all carcinomas of the GEJ could have been included. EC can be coded using “upper third”, “middle third”, or “lower third” in the SEER database. Moreover, EC can be coded by using “cervical esophagus”, “thoracic esophagus”, or “abdominal esophagus”. Carcinoma of GEJ and DE include some of the lower third of the esophagus and some GCs. Also, we did not provide information about how these patients were staged. Finally, we have not got information about toxicity and patterns of failure from the SEER database, as a result, these could not be further analyzed. Last but not least, the use of chemotherapy is not included, which is very important in the treatment regimens.
Despite these limitations, we recognize a number of strengths of our study. Our data was derived from a population-based cohort. The results of our study may be more generalizable to the US population than data from tertiary cancer centers. We had a relatively large sample size with a relatively long period of follow-up, which allowed us to conduct survival analysis and enough power to detect differences between the two groups. Although the SEER Program does not provide data on disease recurrence, it provides cause of death to calculate cancer specific survival, which is correlated with cancer recurrence.
Conclusions
Patients with ESCC of GEJ and DE had a significant worse outcome than that of patients with EAC of GEJ and DE. Surgery is the primary concern for carcinoma of GEJ and DE and neoadjuvant therapy is considerable. Further prospective studies should be performed to confirm the role of neoadjuvant therapy in patients with carcinoma of GEJ and DE and define the optimal delivery of RT.

Full table
Acknowledgments
We would like to express our special thanks to Dr. Wen-Jie Zhang for technical support in this study.
Funding: The study was funded the Natural Science Foundation of China (No. 81272504, No. 81472809), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) (JX10231801), Key Academic Discipline of Jiangsu Province “Medical Aspects of Specific Environments”, Innovation Team [No. LJ201123 (EH11)].
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.03.38). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yee YK, Wong BC. Adenocarcinoma of the esophagogastric junction: do we see more or less? J Gastroenterol Hepatol 2008;23:1627-8. [Crossref] [PubMed]
- Ronellenfitsch U, Schwarzbach M, Hofheinz R, et al. Preoperative chemo(radio)therapy versus primary surgery for gastroesophageal adenocarcinoma: systematic review with meta-analysis combining individual patient and aggregate data. Eur J Cancer 2013;49:3149-58. [Crossref] [PubMed]
- Mariette C, Robb WB, Piessen G, et al. Neoadjuvant chemoradiation in oesophageal cancer. Lancet Oncol 2015;16:1008-9. [Crossref] [PubMed]
- Kleinberg L. Therapy for locally advanced adenocarcinoma of the gastroesophageal junction: optimizing outcome. Semin Radiat Oncol 2013;23:38-50. [Crossref] [PubMed]
- Siewert JR, Hölscher AH, Becker K, et al. Cardia cancer: attempt at a therapeutically relevant classification. Chirurg 1987;58:25-32. [PubMed]
- Siewert JR, Stein HJ, Feith M. Adenocarcinoma of the esophago-gastric junction. Scand J Surg 2006;95:260-9. [Crossref] [PubMed]
- Feith M, Stein HJ, Siewert JR. Adenocarcinoma of the esophagogastric junction: surgical therapy based on 1602 consecutive resected patients. Surg Oncol Clin N Am 2006;15:751-64. [Crossref] [PubMed]
- Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4.
- Rusch VW, Rice TW, Crowley J, et al. The seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Staging Manuals: the new era of data-driven revisions. J Thorac Cardiovasc Surg 2010;139:819-21.
- Gertler R, Stein HJ, Langer R, et al. Long-term outcome of 2920 patients with cancers of the esophagus and esophagogastric junction: evaluation of the New Union Internationale Contre le Cancer/American Joint Cancer Committee staging system. Ann Surg 2011;253:689-98. [Crossref] [PubMed]
- Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer 2010;116:3763-73.
- Ashraf N, Hoffe S, Kim R. Locally advanced gastroesophageal junction tumor: a treatment dilemma. Oncologist 2015;20:134-42. [Crossref] [PubMed]
- Sehdev A, Catenacci DV. Perioperative therapy for locally advanced gastroesophageal cancer: current controversies and consensus of care. J Hematol Oncol 2013;6:66. [Crossref] [PubMed]
- Yoshikawa T, Takeuchi H, Hasegawa S, et al. Theoretical therapeutic impact of lymph node dissection on adenocarcinoma and squamous cell carcinoma of the esophagogastric junction. Gastric Cancer 2016;19:143-9. [Crossref] [PubMed]
- Yabusaki H, Nashimoto A, Matsuki A, et al. Comparison of the surgical treatment strategies for Siewert type II squamous cell carcinoma in the same area as esophagogastric junction carcinoma: data from a single Japanese high-volume cancer center. Surg Today 2014;44:1522-8. [Crossref] [PubMed]
- Arnott SJ, Duncan W, Gignoux M, et al. Preoperative radiotherapy for esophageal carcinoma. Cochrane Database Syst Rev 2005;CD001799 [PubMed]
- Arnott SJ, Duncan W, Gignoux M, et al. Preoperative radiotherapy in esophageal carcinoma: a meta-analysis using individual patient data (Oesophageal Cancer Collaborative Group). Int J Radiat Oncol Biol Phys 1998;41:579-83. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Mullen JT, Kwak EL, Hong TS. What's the Best Way to Treat GE Junction Tumors? Approach Like Gastric Cancer. Ann Surg Oncol 2016;23:3780-5. [Crossref] [PubMed]
- Okines AF, Cunningham D. Multimodality treatment for localized gastro-oesophageal cancer. Ann Oncol 2010;21:vii286-93. [Crossref] [PubMed]
- Mariette C, Piessen G, Briez N, et al. Oesophagogastric junction adenocarcinoma: which therapeutic approach? Lancet Oncol 2011;12:296-305. [Crossref] [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [Crossref] [PubMed]
- Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305-13. [Crossref] [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [Crossref] [PubMed]
- Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005;6:659-68. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851-6. [Crossref] [PubMed]
- Kleinberg LR, Catalano PJ, Forastiere AA, et al. Eastern Cooperative Oncology Group and American College of Radiology Imaging Network Randomized Phase 2 Trial of Neoadjuvant Preoperative Paclitaxel/Cisplatin/Radiation Therapy (RT) or Irinotecan/Cisplatin/RT in Esophageal Adenocarcinoma: Long-Term Outcome and Implications for Trial Design. Int J Radiat Oncol Biol Phys 2016;94:738-46. [Crossref] [PubMed]
- Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014;32:2416-22. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-30. [Crossref] [PubMed]
- Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 2012;30:2327-33. [Crossref] [PubMed]
- Zhu WG, Xua DF, Pu J, et al. A randomized, controlled, multicenter study comparing intensity-modulated radiotherapy plus concurrent chemotherapy with chemotherapy alone in gastric cancer patients with D2 resection. Radiother Oncol 2012;104:361-6. [Crossref] [PubMed]


