
Incidence patterns for myoepithelial carcinoma: a Surveillance, Epidemiology, and End Results (SEER) study
Introduction
Myoepithelial carcinomas are rare malignant tumors arising from myoepithelial cells. It was first described in the salivary glands, and accounts for approximately 1% of all salivary gland tumors (1-5); however, it can also occur in the breast, upper aerodigestive tract, skin, and soft tissue (6-11). Due to its rarity, myoepithelial carcinoma is not well reported and understood, and is usually reported along with myoepithelioma, its more commonly diagnosed benign counterpart. To our knowledge, the largest series for myoepithelial carcinoma published in the English literature included only 40 cases (12), and most literature on myoepithelial carcinoma are individual case reports or small case series (13). There have been no reports with sufficient sample sizes that could enable the analysis of demographics and clinical risk factors which influence its incidence and pattern of occurrence. The lack of such knowledge certainly hampers the understanding of the disease as well as the development of proper diagnostic and treatment techniques. In the present study, therefore, we aimed to determine how age, gender, ethnicity, and the primary tumor location influence the incidence, by analyzing an extensive, population-based sample of both adult and pediatric myoepithelial carcinoma.
Methods
The SEER database was used to select all myoepithelial carcinoma cases from 18 regional cancer registries in the U.S. from 1973 through 2012. All cases of myoepithelial carcinoma diagnosis (ICD-O-3 histology codes 8982) were selected from the registry, and the primary tumor locations were determined by site codes ICD-O-3/WHO 2008.
Age-adjusted incidence rates were determined using the SEER*Stat 8.2.1 program. Differences in the incidence rates between the 2 genders, 3 races, and various tumor locations with the highest frequency (tumor location distinguished by site codes ICD-O-3/WHO 2008) were then compared using a 95% confidence interval (CI). A chi-square test was used to investigate the associations between age, gender, and tumor location by SPSS Version 21.
The age-adjusted rates of disease incidence were determined and analyzed using 95% CIs for a combined incidence rate, which was an estimate for the entire population over the past decade, while Joinpoint analyses were performed using the Joinpoint Regression Program (Version 4.2.0.2, NCI, NIH, USA) to ascertain the annual percentage changes and substantial changes in incidence, respectively.
Results
Three hundred sixty-eight cases were identified from the SEER database by our search criteria. Patients’ characteristics are detailed in Tables 1 and 2. 45.1% of the patients were male and 54.9% of the patients were female; 75% of patients were white and the rest were of other ethnic backgrounds. The median and the mean ages at diagnosis of all patients were 72 and 52.2 years, respectively. Three hundred fifty three (95.9%) patients were adults (>19 years) and 15 (4.1%) were children/adolescents (≤18).

Full table

Full table
Across all age groups, the oral cavity/pharynx were the most common sites of occurrence, followed by breast, soft tissue (including heart), respiratory system, and skin. Age-adjusted incidences (per 100,000 person-years) based on gender, ethnicity, five tumor locations with the highest frequency distinguished by site codes ICD-O-3/WHO 2008 are detailed in Table 3. The incidence was significantly higher (P<0.001) in oral cavity/pharynx as compared to the other four sites, but did not differ significantly with respect to gender or ethnicity (P=0.302 and P=0.119, respectively).
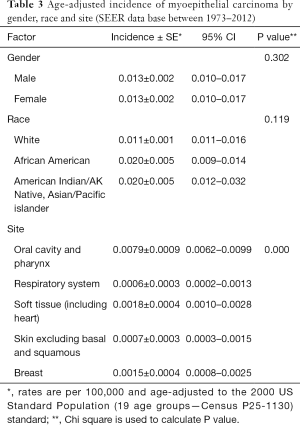
Full table
Subsets of 15 children/adolescents and 327 adult myoepithelial carcinoma were classified by site code into oral cavity/pharynx, respiratory system, soft tissue (including heart), breast, and skin in Table 4. Chi-square analysis that compared age versus disease site showed a statistically significant difference in primary location between adult and pediatric/adolescent patients (P<0.0001). Children/adolescents were significantly more likely to develop myoepithelial carcinoma in the soft tissues, including heart, whereas adult more commonly had oral cavity/pharynx.

Full table
As expected, females were significantly more likely to develop myoepithelial carcinoma in the breast (26.8% vs. 0, Chi-square value =46.52, P<0.001), whereas males more commonly presented in the skin, (7.4% vs. 0.6%, Chi-square value =10.83, P=0.001), oral cavity/pharynx (68.2% vs. 57.0%, Chi-square value =4.363, P=0.037) (Table 5).

Full table
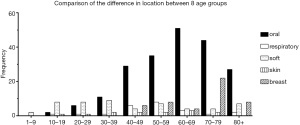
Table 6 and Figure 1 demonstrate the correlations between the age range and the site of diagnosis. The age range of 60–69 has the highest frequency for myoepithelial carcinoma in the oral cavity/pharynx and skin; 70–79 has the highest frequency in breast; and 50–59 has the highest frequency in the respiratory system. The median ages of diagnosis of incidence of skin, oral cavity and pharynx, respiratory system, breast, and soft tissue/heart myoepithelial carcinoma were 55, 65, 55, 75, and 35, respectively.
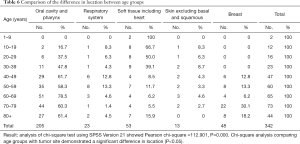
Full table
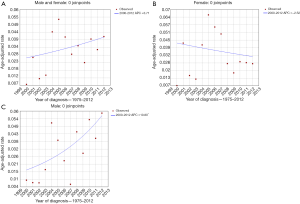
Joinpoint analysis of the entire cohort of patients showed no significant changes in any single year (Figure 2), although we observed a trend of increasing in incidence in both genders. In female patients, the incidence per 100,000 person-years in 2000 and 2012 were 0.007 and 0.025, respectively, and there were no observed percentage changes of statistical significance in annual incidence. However, Joinpoint analysis of male patients showed significant changes in age-adjusted incidence over time. The incidence per 100,000 person-years in 2000 and 2012 were 0.009 vs. 0.058, with an APC (annual percentage change) of 10.8 (P<0.05) in males.
Discussion
Myoepithelial carcinoma is a rare neoplasm composed entirely of myoepithelial cells that exhibit a dual epithelial and smooth muscle phenotype (14). Originally described by Stromeyer et al. in 1975 (15), and further expounded upon by Dardick, these initial reports were crucial in extending the understanding of myoepithelial tumors (8,16). Myoepithelial carcinoma was eventually added to the World Health Organization (WHO) classifications of malignant salivary gland tumors in 1991 (17).
Myoepithelial cells are typically located between the epithelial cells and the basal lamina of acini and ducts of salivary glands, sweat glands, and breasts. Thus myoepithelial neoplasms are more commonly arise in parotid gland (48–75%), followed by minor salivary glands (reported sites include the palate, cheek, gum, nasal cavity, maxillary sinus, nasopharynx, infratemporal fossa, oral cavity, base of tongue, supraglottic larynx), and the submandibular gland. It comprises approximately 1% of all salivary gland carcinomas (11). Occasionally, cases have been reported in the sublingual gland (18-29). Myoepithelial carcinomas diagnosed in other parts of the body are exceedingly rare.
Due to the rarity of myoepithelial carcinoma, its growth patterns, biological behavior, survival data and prognostic factors are largely unknown. The lack of knowledge on the incidence and biological properties of the condition certainly hamper further investigation for the more optimal management of the disease. Therefore, we conducted this study with an aim to describe the basic clinical patterns of the disease occurrence including the most common pathogenic locations, age, differences between adult versus children and male versus female using the SEER.
In our study, 368 patients were collected from the SEER database in 22 different sites over 40 years (Table 1). This is the largest population-based myoepithelial carcinoma distribution study with largest numbers of tumor sites, validating and improving the information described in previous small series or case reports of myoepithelial carcinoma. Across all age groups, oral cavity/pharynx were the most common locations of origin, followed by breast, soft tissue (including heart), respiratory system, and skin where myoepithelial cells are typically located.
Results from previously published series suggested that median age of diagnosis of myoepithelial carcinoma was 60 years (30,31). We had found, similarly, that the median age at diagnosis of all patients was 72 in our series, and the mean age was 52. The age range of 60–69 had the highest frequency for myoepithelial carcinoma in oral cavity and pharynx.
Myoepithelial carcinoma is commonly reported with its benign counterpart, myoepithelioma, in pediatric series; however, it is usually not well represented in most publications. Several authors concluded that they are more aggressive in the pediatric population than in adult patients as half of the cases developed distant metastasis and 43% succumbed from disease progression (8). Because of this apparent difference in biology, patients were stratified by age at the time of their initial diagnosis into children/adolescents (≤18 years) and adults (>19 years) in our study. Only 15 children/adolescents were identified in the database between 1973–2012. Further, we found that adults, particularly males, more commonly had oral cavity/pharynx, which may be induced by more frequent consumption of tobacco and alcohol in adults compared to children, whereas children were more likely to harbor disease arising in soft tissues (including heart), which was highly consistent with the previous reports for myoepithelial carcinoma in children (32,33).
Different single-institution series of myoepithelial carcinoma have showed variability in sex distribution, from equal sex distribution (1:1) to female predominance (4:1) (18,20,22). Our results indicated that the incidence did not differ significantly with respect to sex. Approximately 45% of the patients were male, and 55% were female. The fact that female patients were more likely to harbor myoepithelial carcinoma in the breast as compared to male is not difficult to understand. On the other hand, why the condition occurred more commonly in the skin and oral cavity/pharynx in male patients is perplexing. We speculate that the more frequent use of tobacco and alcohol in males than in females could be the cause of such difference. However, the interesting finding, that the incidence of myoepithelial carcinoma remained stable for females, but demonstrated a trend of increase in male in our series was also puzzling, as the use of tobacco in males has been on a decreasing trend in the U.S. in the past several decades. The underlying mechanism for the increasing trend of myoepithelial carcinoma in males remains unknown. Among all the ethnic groups in the U.S., the predominant race was White as compared to other races including African American, American Indian/Alaskan Native, and Asian as expected because of the overall racial compositions of U.S., no difference in incidence were demonstrated by race of the patients.
The SEER data is inherently limited due to the voluntary nature of data collection, and represents only 28% of the U.S. cancer patient population. The two previous reports on pediatric myoepithelial carcinoma had 36 patients combined (32,33). And the fact that only 15 patients in our SEER analysis were younger than 19 clearly illustrated the incompleteness of the data on pediatric patients. Nevertheless, the majority of the patients in the two reports on pediatric myoepithelial carcinoma had soft tissue myoepithelial carcinoma. Such findings were highly consistent with our results.
Ideally, the analysis of a rare event, such as myoepithelial carcinoma, should be analyzed from a more complete dataset that is gathered over an effective period of time and is further subjected to variability from different suppliers of data. However, changes in the diagnostic criteria in the past few decades can also result in the under-reporting of myoepithelial carcinoma cases in the SEER registry. The diagnostic process is typically done locally and information including immunohistochemistry or electron microscopic confirmation of the diagnosis is not always available. Even with complete information, definite pathological criteria of myoepithelial carcinoma are not always well delineated. Although we tried to analyze the data collected between 1973–2012, the missing details, especially those in the earlier years, make it difficult to accurately analyze the trend of the disease over a longer period of time. As a result, we could only get the trend of incidence patterns over the last decade. Despite all of the aforementioned limitations, this series is the largest study to analyze the incidence patterns of patients with myoepithelial carcinoma and may prove useful in providing insight and directing future investigations on patient outcomes.
Conclusions
This SEER analysis is the largest observational study that examines the associations of age at diagnosis, gender, ethnicity, and primary tumor location and correlates with the incidence of myoepithelial carcinoma. The incidence was higher in oral cavity/pharynx than other sites, but did not differ in overall incidence with respect to gender or ethnicity. The median age of diagnosis was 70 years. In addition, our study demonstrated that the overall incidence of myoepithelial carcinoma seems to have increased over the last decade. Such an observation is largely driven by the male patient population. Understanding these incidence patterns with respect to the location of the disease and age may facilitate in the advancement our understanding of the biology of myoepithelial carcinoma and its management.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.04.15). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This article does not contain any studies with human participants performed by any of the authors. Institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sciubba JJ, Brannon RB. Myoepithelioma of salivary glands: report of 23 cases. Cancer 1982;49:562-72. [Crossref] [PubMed]
- Barnes L, Appel BN, Perez H, et al. Myoepitheliomas of the head and neck: case report and review. J Surg Oncol 1985;28:21-8. [Crossref] [PubMed]
- Dardick I, Thomas MJ, Nostrand AW. Myoepitheliomanew concepts of histology and classification: a light and electron microscopic study. Ultrastruct Pathol 1989;13:187-224. [Crossref] [PubMed]
- Seifert G, Sobin L. Histological typing of salivary gland tumors (World Health Organization). 2nd edition. New York: Springer-Verlag, 1991.
- Ellis GL, Auclair PL. Tumors of the salivary glands. Washington, DC: Armed Forces Institute of Pathology, 1996.
- Sheldon W. So-called mixed tumors of the salivary glands. Arch Pathol 1943;35:1-20.
- Hornick JL, Fletcher CD. Myoepithelial tumors of soft tissue: a clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol 2003;27:1183-96. [Crossref] [PubMed]
- Kilpatrick SE, Hitchcock MG, Kraus MD, et al. Mixed tumors and myoepitheliomas of soft tissue: a clinicopathologic study of 19 cases with a unifying concept. Am J Surg Pathol 1997;21:13-22. [Crossref] [PubMed]
- Fletcher CD, Unni KK, Mertens F. editors. Pathology and Genetics of Tumours of Soft Tissue and Bone. World Health Organization Classification of Tumours. Lyon: IARC Press, 2002:198-99.
- Michal M, Miettinen M. Myoepitheliomas of the skin and soft tissues. Report of 12 cases. Virchows Arch 1999;434:393-400. [Crossref] [PubMed]
- Yoshizaki T, Himi Y, Minato H, et al. Malignant myoepithelioma arising from recurrent pleomorphic adenoma of minor salivary gland. Auris Nasus Larynx 2002;29:91-4. [Crossref] [PubMed]
- Hornick JL, Flectcher CD. A clinicopathologic and immunohistochemical study of 101 cases with evaluation of prifnostic parameters. Am J Surg Pathol 2003;27:1183-96. [Crossref] [PubMed]
- Hornick JL, Fletcher CD. Myoepithelial tumors of soft tissue a clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol 2003;27:1183-96. [Crossref] [PubMed]
- Barnes LEJ, Reichart P, Sidransky D. Pathology and Genetics Head and Neck Tumors. Lyon: IARC Press, 2005:240-1.
- Stromeyer FW, Haggitt RC, Nelson JF, et al. Myoepithelioma of minor salivary gland origin. Light and electron microscopical study. Arch Pathol 1975;99:242-5. [PubMed]
- Dardick I. Myoepithelioma: definitions and diagnostic criteria. Ultrastruct Pathol 1995;19:335-45. [Crossref] [PubMed]
- Seifert G, Sobin LH. The World Health Organization's Histological Classification of Salivary Gland Tumors. A commentary on the second edition. Cancer 1992;70:379-85.
- Savera AT, Sloman A, Huvos AG, et al. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 25 patients. Am J Surg Pathol 2000;24:761-74. [Crossref] [PubMed]
- Di Palma S, Guzzo M. Malignant myoepithelioma of salivary glands: clinicopathological features of ten cases. Virchows Arch A Pathol Anat Histopathol 1993;423:389-96. [Crossref] [PubMed]
- Skalova A, Jakel KT. Tumours of the salivary glands. Barnes L, Eveson JW, Reichart P, Sidransky D. WHO Classification of Tumours;Pathology and genetics of Head and Neck Tumours. Lyon, France: IARC Press, 2005:240-1.
- Nagao T, Sugano I, Ishida Y, et al. Salivary gland malignant myoepithelioma: a clinicopathologic and immunohistochemical study of ten cases. Cancer 1998;83:1292-9. [Crossref] [PubMed]
- Yu G, Ma D, Sun K, et al. Myoepithelial carcinoma of the salivary glands: behavior and management. Chin Med J (Engl) 2003;116:163-5. [PubMed]
- Nayak JV, Molina JT, Smith JC, et al. Myoepithelial neoplasia of the submandibular gland: case report and therapeutic considerations. Arch Otolaryngol Head Neck Surg 2003;129:359-62. [Crossref] [PubMed]
- Tuncel U, Ergul G, Ozlugedik S, et al. Myoepithelial carcinoma in the nasopharynx: an unusual localization. Yonsei Med J 2004;45:161-5. [Crossref] [PubMed]
- Ghosh A, Saha S, Saha VP, et al. Infratemporal fossa myoepithelial carcinoma-a rare case report. Oral Maxillofac Surg 2009;13:59-62. [Crossref] [PubMed]
- Herrera GA. Light microscopic, ultrastructural and immunocytochemical spectrum of malignant lacrimal and salivary gland tumors, including malignant mixed tumors. Pathobiology 1990;58:312-22. [Crossref] [PubMed]
- Nilles R, Lenarz T, Kaiserling E. (Myoepithelial carcinoma of the nasopharynx. Case report and review of the literature). HNO 1993;41:396-400. [PubMed]
- Ibrahim R, Bird DJ, Sieler MW. Malignant myoepithelioma of the larynx with massive metastatic spread to the liver: an ultrastructural and immunocytochemical study. Ultrastruct Pathol 1991;15:69-76. [Crossref] [PubMed]
- Losito NS, Botti G, Ionna F, et al. Clear-cell myoepithelial carcinoma of the salivary glands: a clinicopathologic, immunohistochemical, and ultrastructural study of two cases involving the submandibular gland with review of the literature. Pathol Res Pract 2008;204:335-44. [Crossref] [PubMed]
- Gnepp D, Brandwein M, Henley J. Chapter 6: Salivary and lacrimal glands. In: Gnepp DR. editor. Diagnostic surgical pathology of the head and neck. Philadelphia: W.B. Saunders Company, 2001.
- Wang J, Wu Q, Sun K, et al. Quantitative multivariate analysis of myoepithelioma and myoepithelial carcinoma. Int J Oral Maxillofac Surg 1995;24:153-7. [Crossref] [PubMed]
- Gleason BC, Fletcher CD. Myoepithelial Carcinoma of Soft Tissue in Children: An Aggressive Neoplasm Analyzed in a Series of 29 Cases. Am J Surg Pathol 2007;31:1813-24. [Crossref] [PubMed]
- Bisogno G, Tagarelli A, Schiavetti A. Myoepithelial carcinoma treatment in children: a report from the TREP project. Pediatr Blood Cancer 2014;61:643-6. [Crossref] [PubMed]


