
Concomitant ALK/KRAS and ALK/EGFR mutations in non small cell lung cancer: different profile of response to target therapies
The development of therapeutic agents targeting products of epidermal growth factor receptor (EGFR) gene mutation and anaplastic lymphoma kinase (ALK) rearrangements has significantly improved survival in patients with non small cell lung cancer (NSCLC). Thus, the patients eligible for the treatment with EGFR or ALK inhibitors should be selected through appropriate molecular tests (1). On the other hand, although representing the most frequent genic alteration in NSCLC patients, Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation-specific therapy has not been validated in clinical practice (2). Indeed KRAS mutations are described in approximately 20–30% of NSCLC, commonly observed in smokers and associated with a poor prognosis (2).
Although driver genes mutations were reported to be mutually exclusive in NSCLC (3,4), however in several series driver genes mutations seem to occur particularly associated to EGFR mutations (5,6). In wide series of NSCLC, rare cases of concomitant mutations were reported with different frequency, however the TKI response data were conflicting (6-8). In particular, the frequency of concurrent EGFR/ALK mutations was reported in a range of 0.0% to 6% (6,9). Recently, in a large series of Chinese NSCLC patients, the concomitant EGFR and ALK mutations was observed in 1.9% of the cases (6). In a total of 977 NSCLCs, EGFR mutations was found in 336 (32.7%), ALK rearrangements in 70 (6.8%), KRAS mutation in 40 (3.9%) patients and concomitant EGFR and ALK aberrations were observed in 13 patients (1.3%). Although the overall rate of EGFR/ALK co-alterations was only of 1.3% (13/977), however the prevalence of co-alterations was 3.9% (13/336) in EGFR mutant patients and 18.6% (13/70) in ALK-positive patients (6). These results suggested that driver mutations of EGFR and ALK genes could occur in a small group of NSCLC, but more frequently in ALK-positive tumors. In literature, the concomitant EGFR/KRAS mutations were described mainly in case reports, but lately in a large cohort of 5,125 Chinese NSCLCs 30 cases harboring concomitant aberrations were reported (5).
Besides ALK and KRAS alterations, several mutations in various oncogenes were described as concomitant with EGFR mutations. Rarely occurrence of other driver genes mutations were reported associated to EGFR mutations, such as HER2, RET, KRAS and ROS1 genes mutations, while no BRAF and NRAS were found coexisting with EGFR mutations (6). Furthermore, the phosphatidylinositol 3-kinase (PI3K), playing a critical role in cancer cell proliferation, is mutated in approximately 2–4% of NSCLCs (10), often associated to KRAS mutations and less frequently with EGFR and ALK mutations (11).
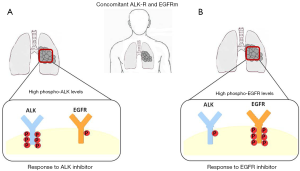
The concomitant EGFR mutations and other driver genes might decrease substantially the efficacy of EGFR-TKIs (6). The median PFS of patients with concurrent EGFR/ALK mutations treated with EGFR-TKI ranged from 5.0 to 11.2 months, relatively lower than patients harboring only EGFR mutation (6,7). Ulivi et al. observed disease control rate (DCR) in 67% of co-altered patients, that is lower than the 81.7% in patients with an EGFR-mutation only (12). Particularly Yang et al. attributed the efficacy of EGFR-TKI to relative levels of phospho-EGFR in patients with concomitant EGFR/ALK mutations (7). Indeed of the ten patients receiving first-line EGFR-TKIs, eight achieved objective response with a median PFS of 11.2 months. Of the four patients treated with crizotinib, three cases were previously treated with EGFR-TKI, particularly one case was not responsive to EGFR-TKI, but sensitive to crizotinib, whilst two cases were responsive to EGFR-TKI, but not to crizotinib. Finally, one case showed partial response to crizotinib, but no response to subsequent treatment with EGFR-TKI. Immunohistochemistry showed in all examined cases co-expression of EGFR mutant protein and ALK fusion proteins in the same cancer cells, indicating that different driver oncogenes could act in the same cell population. Moreover, different levels of receptors phosphorylation were observed using specific antibodies. Thus, three patterns of phosphorylated proteins were documented: high p-EGFR and high p-ALK, high p-EGFR and low p-ALK, and low p-EGFR and high p-ALK. High levels of p-EGFR correspond to partial responses to EGFR-TKI, while two patients with low levels of p-EGFR had progressive or stable disease. Of the four cases treated with crizotinib, two had low p-EGFR and high p-ALK; one of them was not responder to EGFR-TKI, but sensitive to crizotinib, and the other was highly responsive to crizotinib, but resistant to subsequent EGFR-TKI. On the other hand, two cases had high p-EGFR levels and low p-ALK levels, corresponding to partial responsiveness to EGFR-TKI, but with poor results when treated with crizotinib (7).
Generally, the results of subsequent treatment with crizotinib in NSCLC patients with concomitant EGFR/ALK mutations after failure of EGFR-TKI treatment are conflicting (6). Lee et al. observed that two ALK-positive/EGFR-mutant NSCLC patients non-responder to EGFR-TKI showed a durable partial response to ALK inhibitors (13). Therefore, in non-responded to EGFR-TKI patients, ALK gene status test should be investigated, since it might be responsible for unsuccessful treatment. In parallel, acquired EGFR mutations are also described as a mechanism of resistance to ALK inhibitor (14). However, in a series of 1,683 NSCLCs, all 25 ALK-positive patients crizotinib-resistant were both KRAS and EGFR wild type (3).
Finally, in NSCLC patients harboring ALK/EGFR co-alterations, EGFR-TKIs seem to be more active compared to ALK-TKIs. Schmid et al. identified five patients with EGFR/ALK co-alteration, four out of five received one or more lines of EGFR-TKIs and three patients received one or more lines of ALK-TKI. In particular, patients showed different response to TKI: one out of three patients responding to ALK-TKI and three out of four patients responding to EGFR-TKI. Median PFS were slightly better in patients treated with EGFR-TKIs than in patients treated with ALK-TKIs (8).
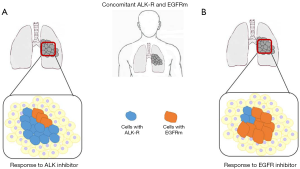
Different response rate might be explained considering intratumor heterogeneity of both genes, strictly related to gene mutation tumor burden (9,15). Therefore, the mutation tumor burden of each mutation could affect targeted therapy response. Won et al. detected in a series of 1,458 NSCLC 14 EGFR/ALK co-altered cases, eight patients treated with crizotinib showed DCR and three patients who received EGFR-TKI showed poor response. These results could be explained considering that most patients were studied for EGFR mutations through targeted NGS or mutant-enriched NGS, suggesting that relative lower EGFR-mutation burden could cause lack of response to EGFR-TKIs in these patients (16). Thus, since highly sensitive EGFR tests have widely been introducing in practical diagnosis of NCSLC, an increasingly high number of cases with concomitant alterations in different oncogenes could be identified in the future. It is calculated a significant increased rate of concomitant EGFR and ALK mutations in NSCLC—from 4.4% to 15.4%—using targeted next-generation sequencing (NGS) (16).
As regards KRAS mutations, since they are responsible for secondary resistance to ALK TKIs such as crizotinib (17), the concomitant ALK and KRAS co-alteration may be associated with primary resistance to ALK-TKI treatment. Indeed, for the first time Schmid et al. reported that six out of seven patients treated with crizotinib were non-responder patients (8). In the clinical setting of concomitant EGFR/KRAS mutations, KRAS mutations seems to be related to a reduced response to EGFR TKI (2). On the contrary Lee et al. reported that response rate to EGFR-TKI in concomitant EGFR/KRAS mutation patients is superimposable to only EGFR mutant patients. This observation might be attributable to EGFR mutation as driver dominant role, even if the tumor cells harbored an additional KRAS mutation (14).
In conclusion, most NSCLC patients harboring concomitant EGFR/KRAS mutations partially responded to EGFR TKI, while NSCLC patients harboring concomitant EGFR/ALK mutations slightly responded to specific ALK or EGFR TKI. EGFR and ALK alterations play an important role in the oncogenesis of NSCLC, however their interaction in terms of synergism versus the possible dominance of one rather than the other oncogene are currently not completely clarified. The dominance of one oncogenic alteration over the other could be explained essentially through two mechanisms, a different mutation tumor burden of each driver gene (Figure 1) and differential phosphorylation of the single mutated proteins (Figure 2). Different mutation tumor burden could justify the inconsistency of TKI response in patients investigated through cytology or small biopsies, clearly representing only a small portion of the entire tumor. On the other hand the presence of concomitant EGFR/ALK mutation could have a little value, if not associated to the evaluation of altered protein phosphorylation. Finally, the alternative over-phosphorylation of altered EGFR and ALK proteins needs more studies of validation in order to address the patients to the better treatment.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shaohua Cui (Department of Pulmonary Medicine, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.03.77). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch Pathol Lab Med 2013;137:828-60. [Crossref] [PubMed]
- Roberts PJ, Stinchcombe TE. KRAS mutation: should we test for it, and does it matter? J Clin Oncol 2013;31:1112-21. [Crossref] [PubMed]
- Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res 2013;19:4273-81. [Crossref] [PubMed]
- Zhang X, Zhang S, Yang X, et al. Fusion of EML4 and ALK is associated with development of lung adenocarcinomas lacking EGFR and KRAS mutations and is correlated with ALK expression. Mol Cancer 2010;9:188. [Crossref] [PubMed]
- Li S, Li L, Zhu Y, et al. Coexistence of EGFR with KRAS, or BRAF, or PIK3CA somatic mutations in lung cancer: a comprehensive mutation profiling from 5125 Chinese cohorts. Br J Cancer 2014;110:2812-20. [Crossref] [PubMed]
- Hu W, Liu Y, Chen J. Concurrent gene alterations with EGFR mutation and treatment efficacy of EGFR-TKIs in Chinese patients with non-small cell lung cancer. Oncotarget 2017; [Epub ahead of print]. [PubMed]
- Yang JJ, Zhang XC, Su J, et al. Lung cancers with concomitant EGFR mutations and ALK rearrangements: diverse responses to EGFR-TKI and crizotinib in relation to diverse receptors phosphorylation. Clin Cancer Res 2014;20:1383-92. [Crossref] [PubMed]
- Schmid S, Gautschi O, Rothschild S, et al. Clinical Outcome of ALK-Positive Non-Small Cell Lung Cancer (NSCLC) Patients with De Novo EGFR or KRAS Co-Mutations Receiving Tyrosine Kinase Inhibitors (TKIs). J Thorac Oncol 2017;12:681-8. [Crossref] [PubMed]
- Zito Marino F, Liguori G, Aquino G, et al. Intratumor Heterogeneity of ALK-Rearrangements and Homogeneity of EGFR-Mutations in Mixed Lung Adenocarcinoma. PLoS One 2015;10:e0139264 [Crossref] [PubMed]
- Scheffler M, Bos M, Gardizi M, et al. PIK3CA mutations in non-small cell lung cancer (NSCLC): genetic heterogeneity, prognostic impact and incidence of prior malignancies. Oncotarget 2015;6:1315-26. [Crossref] [PubMed]
- Chaft JE, Arcila ME, Paik PK, et al. Coexistence of PIK3CA and other oncogene mutations in lung adenocarcinoma-rationale for comprehensive mutation profiling. Mol Cancer Ther 2012;11:485-91. [Crossref] [PubMed]
- Ulivi P, Chiadini E, Dazzi C, et al. Nonsquamous, Non-Small-Cell Lung Cancer patients who carry a double mutation of EGFR, EML4-ALK or KRAS: frequency, clinical-pathological characteristics, and response to therapy. Clin Lung Cancer 2016;17:384-90. [Crossref] [PubMed]
- Lee JK, Kim TM, Koh Y, et al. Differential sensitivities to tyrosine kinase inhibitors in NSCLC harboring EGFR mutation and ALK translocation. Lung Cancer 2012;77:460-3. [Crossref] [PubMed]
- Lee T, Lee B, Choi YL, et al. Non-small Cell Lung Cancer with concomitant EGFR, KRAS, and ALK Mutation: clinicopathologic features of 12 Cases. J Pathol Transl Med 2016;50:197-203. [Crossref] [PubMed]
- Zito Marino F, Rossi G, Brunelli M, et al. Diagnosis of anaplastic lymphoma kinase rearrangement in cytological samples through a fluorescence in situ hybridization-based assay: Cytological smears versus cell blocks. Cancer 2017; [Epub ahead of print]. [PubMed]
- Won JK, Keam B, Koh J, et al. Concomitant ALK translocation and EGFR mutation in lung cancer: a comparison of direct sequencing and sensitive assays and the impact on responsiveness to tyrosine kinase inhibitor. Ann Oncol 2015;26:348-54. [Crossref] [PubMed]
- Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 2012;18:1472-82. [Crossref] [PubMed]


