
Targeted therapy in small cell lung cancer: can DLL3 notch up a victory?
Introduction
Small cell lung cancer (SCLC) is the most lethal and aggressive subtype of lung carcinoma, responsible for ~13–18% of lung cancer death with no appreciable improvements in outcomes or treatment options for the last 30 years. The clinical behavior of SCLC is tailor made for nihilism with excellent initial overall response rates transforming to inevitable chemotherapy resistant recurrence in the majority of patients. Targeted therapies to date have failed with little to no efficacy in unselected populations. Naturally, this state of affairs has led to an underfunded SCLC research community, and historical pharmaceutical disinterest in this “graveyard of drug development”. The National Cancer Institute (NCI) and worldwide refocus upon “recalcitrant” carcinomas has led to renewed interest in SCLC making this the perfect opportunity to consider how and why targeted therapy in unselected SCLC has failed so consistently. The critical factors are both biological factors and structural limitations to previous targeted therapy studies in SCLC. (I) The rapid recurrence after initial response to chemotherapy of SCLC is suggestive of biological features consistent with stem cell biology. This strongly suggests a stem cell like phenotype, or a resistant subclonal expansion (1). Stem cell signaling is complex and redundant which limits signaling interference as a monotherapy; (II) the lack of mutational drivers and the mutational signature of SCLC appears to be principally driven by changes in tumor suppressor or transcription factors. These targets are challenging to drug and this has hampered targeted therapy options; (III) the related issue of inadequate biomarkers for the delineation of SCLC subpopulations. SCLC has long been known to be a heterogeneous disease (2), but previously the tools were unavailable to further characterize potential subpopulations by single cell based methods. The study “Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study” recently published in Lancet Oncology constitutes an attempt to address these critical factors in SCLC biology: stem cell targeting, lack of a novel druggable target, and biomarker driven clinical trials. This study is a promising theoretical approach using an antibody drug conjugate (ADC) to target DLL3 labeled putative stem cell populations in SCLC and incorporates an intrinsic biomarker of response.
Theoretical underpinnings: SCLC and the stem cell hypothesis
The putative cell of origin for SCLC is the pulmonary neuroendocrine cell (PNEC) which participates in oxygen sensing and lung morphogenesis. This cell of origin has not been definitively established in human cancers, but multiple SCLC murine models implicate p53 and Rb loss in the neuroendocrine cell niche (3). These PNECs have a substantial stem cell and injury repair role in normal physiology and have stem cell like properties including transdifferentiation capability (4). The maintenance of this injury repair capability relies on the contribution of multiple signaling pathways including the Hedgehog (Hh) pathway (5) and Notch activation inhibits the related processes of epithelial-mesenchymal transition (EMT) and invasion (6). These same signaling pathways along with SOX2 and MYCL1 are vital to the maintenance and growth of SCLC tumors (7).
SCLC, druggable targets and biomarkers
Recent genetic analyses from multiple groups have expanded our understanding of the underlying gene expression associated with SCLC and have identified putative “stemness” signaling targets in SCLC (7,8). These studies have uncovered changes in multiple pathways with readouts amenable to biomarker or mutational analysis including SHH, PTEN, NOTCH, EZH2, FGFR and others (Table 1). However, given the high mutational burden in SCLC, it remains unclear the relative contributions of each biomarker and the precise delineation of passenger and driver mutations in SCLC. The largest analysis to date involved sequencing data from 152 primary tumor specimens and RNAseq analysis on a subset of 81 primary tumors (8). One notable pathway implicated from this study was notch signaling which is downregulated in 77% of SCLC tumors (8). NOTCH family genes had genomic alterations in 25% of SCLC tumors. Additional studies found reduced tumor formation, metastatic capability, cell cycle inhibition, and reduced neuroendocrine markers with Notch activation thus demonstrating NOTCH as a tumor suppressor in SCLC (6,8).
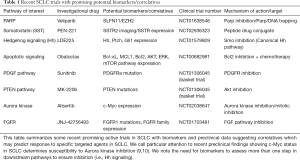
Full table
Saunders et al.’s pre-clinical findings expand upon this work by focusing on DLL3, an inhibitory notch ligand which was found to be over expressed in both patient derived xenografts (PDX) and a cohort of primary SCLC tumors (11). This inhibitory ligand is downstream of the ASCL1 neuroendocrine differentiation pathway and has high level surface expression in SCLC and LCNEC tumors, but low expression in normal lung tissue and normal expression confined largely to the brain. This combination of characteristics made DLL3 an ideal candidate for an ADC with the advantage that DLL3 expression thereby formed an intrinsic predictive biomarker for response. Pre-clinical results supported this hypothesis and showed that DLL3 expression in the PDX model was predictive of response to the ADC with multiple high DLL3 expressing PDX showing complete responses and xenograft rejection (11). Additional work has also described a potential role for future “theragnostic” approaches allowing for the noninvasive imaging of DLL3 status (12). These data provided compelling pre-clinical evidence for efficacy leading to the first successful targeted therapy study in SCLC.
Clinical/practice impact of this study
It is important to note that this is a phase 1 study with a primary focus on safety and tolerability. The expansion cohort and planned phase 2 arm of the study branched off into NCT 02674568. Toxicity was not insignificant at the intended phase 2 dose with a relatively novel toxicity pattern of serosal effusions including serious pleural and pericardial effusion requiring paracentesis. Grade 3–4 thrombocytopenia was also noted in 11% of patients. The etiology most likely is an off-target effect from the conjugate toxin based on what is currently known about the expression pattern of DLL3. However, these toxicities are manageable with clinical awareness and compare reasonably with alternative potential agents (13), although they may be a concern in an already frail patient population receiving third line therapy.
In this phase I trial, of the 60 patients who received therapeutic dose levels of Rova-T, there was an 18% response rate which is comparable with existing second line agents. However, it is worth noting that among patients with at least 50% DLL3+ tumor tissue there was a more impressive 38% response rate with a disease control rate of 85% and a PFS of 4.6 months. For this extensively treated patient population with limited therapeutic options, this could be considered clinically significant. Moreover, among the responding patients, there are multiple patients who had responses of greater than 12 months with multiple patients still alive post study completion.
This is very exciting and suggests both a strong predictive effect of the DLL3 expression and strong clinical potential for Rova-T given the lack of options beyond first line therapy for SCLC patients. We should note the obligatory caveats of preliminary results from a small study with a select patient population, but overall this study is well designed with an excellent predictive intrinsic biomarker and promising clinical activity. We await confirmation from larger phase II/III trials where careful monitoring of the novel toxicities associated with this agent will be needed.
Additionally, future phase II and III studies should incorporate post treatment DLL3 analysis or other Notch/neuroendocrine identity components to better identify mechanisms of resistance including DLL3 downregulation or alternative pathways. Similarly to the approach with targeted therapy in NSCLC patients, the acquisition of rational molecular correlates and clinical samples upfront in studies is increasingly important in order to anticipate future mechanisms of resistance and design trials appropriately to treat this highly heterogeneous and challenging carcinoma.
Conclusions
SCLC is a highly aggressive and heterogeneous lung cancer where targeted therapies have lagged behind. However, the primary clinical approach to date has used unselected SCLC patient populations. This is suboptimal and has stemmed from the lack of genetic and expression information on SCLC and the extreme heterogeneity of this tumor. The recent study “Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study”, demonstrates how a trial approach incorporating an intrinsic biomarker targeting a specific stem cell like population can have efficacy, even in 3rd line+ therapy in SCLC. SCLC clinical research needs to move in the direction of biomarker driven selected population or unselected populations with appropriate and extensive correlates in order to identify and treat the right patient with the right drug at the right time.
Acknowledgments
JM Lehman is provided by a Career Development Award from the LUNGevity foundation.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shaohua Cui (Department of Pulmonary Medicine, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.03.70). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Codony-Servat J, Verlicchi A, Rosell R. Cancer stem cells in small cell lung cancer. Transl Lung Cancer Res 2016;5:16-25. [PubMed]
- Carney DN, Gazdar AF, Nau M, et al. Biological heterogeneity of small cell lung cancer. Semin Oncol 1985;12:289-303. [PubMed]
- Park KS, Liang MC, Raiser DM, et al. Characterization of the cell of origin for small cell lung cancer. Cell Cycle 2011;10:2806-15. [Crossref] [PubMed]
- Song H, Yao E, Lin C, et al. Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc Natl Acad Sci U S A 2012;109:17531-6. [Crossref] [PubMed]
- Watkins DN, Berman DM, Burkholder SG, et al. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature 2003;422:313-7. [Crossref] [PubMed]
- Hassan WA, Yoshida R, Kudoh S, et al. Notch1 controls cell invasion and metastasis in small cell lung carcinoma cell lines. Lung Cancer 2014;86:304-10. [Crossref] [PubMed]
- Rudin CM, Durinck S, Stawiski EW, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet 2012;44:1111-6. [Crossref] [PubMed]
- George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47-53. [Crossref] [PubMed]
- Mollaoglu G, Guthrie MR, Böhm S, et al. MYC Drives Progression of Small Cell Lung Cancer to a Variant Neuroendocrine Subtype with Vulnerability to Aurora Kinase Inhibition. Cancer Cell 2017;31:270-85. [Crossref] [PubMed]
- Helfrich BA, Kim J, Gao D, et al. Barasertib (AZD1152), a Small Molecule Aurora B Inhibitor, Inhibits the Growth of SCLC Cell Lines In Vitro and In Vivo. Mol Cancer Ther 2016;15:2314-22. [Crossref] [PubMed]
- Saunders LR, Bankovich AJ, Anderson WC, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med 2015;7:302ra136 [Crossref] [PubMed]
- Sharma SK, Pourat J, Carlin S, et al. A DLL3-targeted theranostic for small cell lung cancer: Imaging a low density target with a site-specifically modified radioimmunoconjugate. J Nucl Med 2016;57:50.
- Horita N, Yamamoto M, Sato T, et al. Topotecan for Relapsed Small-cell Lung Cancer: Systematic Review and Meta-Analysis of 1347 Patients. Sci Rep 2015;5:15437. [Crossref] [PubMed]


