
Getting to know ovarian cancer spheroid: opportunity for spheroid-targeted therapy
Epithelial ovarian cancer (EOC) is the most lethal gynecological cancer among women (1). Despite great improvement in cancer therapy during past decade, survival of EOC remains disappointing with overall 5-year survival rate of approximate 46%. The high lethality of EOC is attributed to the rapid and early metastasis and lack of early detection. Seventy percent of EOC patients were diagnosed in advanced stage disease (FIGO III or IV) thus losing the opportunity of curative surgery (2). For these patients, cytoreductive surgery plus adjuvant chemotherapy is the last treatment option. Nevertheless, although most patients initially respond to current chemotherapeutic regimes, relapse common with recurrent rate of approximately 75% within 3 years (3). Therefore, it is imperative to reveal the mechanism underlying EOC metastasis and drug resistance for new treatment strategy development and improvement of clinical outcomes.
The primary metastasis mode of EOC is transcoelomic which involves tumor cell detached from primary tumor, transported and implanted in peritoneum and pelvic organs. In this process, detached tumor cells aggregate as spheroid within the abdominal cavity to overcome anoikis. These spheroids can spread over the peritoneal cavity, invade the peritoneum and implant in pelvic organs and omentum. In this context, spheroid formation is a key step in transcoelomic that facilitate detached tumor cells to maintain cell-cell contact and support anchorage-independent tumor growth (4). Besides, the 3D-structured spheroids are widely recognized as the source of drug resistance to current anti-apoptotic small molecules. However, the mechanisms underlying the chemoresistance of tumor spheroid are not clear.
Accumulating evidences suggest that presence of cancer stem cells (CSCs) in spheroid is accounted for chemoresistance in EOCs. CSCs are a group of progenitor cells that possess stem cell-like characteristics including self-renewal, high proliferative, and differentiation into multiple cell types, etc. CSCs are widely thought to be a source of metastasis, recurrence and drug resistance in many malignancies. In EOC, studies have showed spheroids were enriched for cells with CSC characteristics such as upregulation of stem cell genes, self-renewal, high aldehyde dehydrogenase (ALDH) activity, and more aggressive growth pattern (5-7). Genomic signature analysis identified several CSC markers that were upregulated in spheroids, including ALDH1A1, β-catenin, and c-KIT (5). Similarly, epithelial-mesenchymal transition (EMT) spectrum can define a steroidogenic intermediate mesenchymal state, and such EMT gene expression signatures correlate with worse clinical outcomes. These EMT and CSC-like phenotypes may facilitate chemoresistance in recurrent EOC (8). These data strongly support the concept that EOC cells that form spheroids are enriched for CSCs, allowing transcoelomic metastasis and persistence after chemotherapy. Therefore, agents targeting cancer stem-like cells in spheroid may have promise potential in overcoming drug resistance in OC treatment.
Realizing the importance of CSCs, lots of efforts have been devoted to uncovering the mechanism of maintaining CSCs-like phenotype in spheroid. Liao et al. revealed that EOC spheroid cells route glucose predominantly to anaerobic glycolysis and pentose cycle to the detriment of re-routing glucose for anabolic purposes (7). Because many drugs like angiogenesis inhibitors kill tumor cells by cutting off the blood and oxygen supply, these metabolic properties of spheroid confer increased resistance to hypoxia and hence a selection advantage, and eventually generation cancer stem-like cells with drug resistance and more aggressive phenotype (9).
A recent study by Chen et al. has made a great stride in identifying the signal transducer and activator of transcription-3 (STAT3)-mediated stem cell pathway, which is critical for EOC spheroid formation, tumorigenicity and chemo-resistance. First, they found that significant higher levels of STAT3 phosphorylation in ovarian cancer spheroids from 31 recurrent tumors compared to paired primary tumor tissues. Similarly, phosphorylation of STAT3 was increased when EOC cells were grown in 3-dimensional suspension cultures compared with 2-dimensional adherent cultures in a panel of EOC cell lines (SKOV3, HeyA8 and ES2). STAT3 deprivation significantly impaired spheroid formation, attenuated stem-cell like characteristics, and conveyed to reduced chemoresistance in vitro 3D culture and in vivo mouse models (10). Interestingly, in the mouse EOC models, paclitaxel-treated group decreased bulk tumor (>3 mm) seedings, but not small-tumor seeding (≤1 mm); STAT3-knockdown group only inhibited small-tumor seeding; combination of STAT3-knockdown and paclitaxel group obviously diminished all size of tumors. Their data support that STAT3 is crucial for initiation of EOC spheroid formation and CSC-like phenotype. The synergistic effect has great clinical implications and calls for future work to evaluate the clinical benefit of targeting STAT3 in combination with paclitaxel in treatment advanced OC (10).
Wnt/β-catenin pathway is regarded as CSCs regulator in many different organs and plays a key role in cell transformation and tumor progression of EOC (11). Activation of β-catenin regulates the tumor-initiating capacity and spheroid formation in EOC (5). Consistently, Chen and colleague found that Wnt antagonist DKK1 was the most significantly upregulated gene in response to STAT3 knockdown, while silencing of DKK1 remarkably reversed the sphere-forming ability. Further mechanistic study found STAT3 unregulated miR-92a expression level, the later binds to 3’UTR of DKK1 and inhibits DKK1 transcription. Both in vitro and in vivo study demonstrated that STAT3 knockdown-induced reduction in spheroid formation, CSC phenotype, and tumorigenicity could be recovered by overexpression of miR-92a. Taken together, this study elegantly defines a STAT3-miR-92a-DKK1-Wnt stem pathway in the generation of cancer stem-like cells in ovarian tumors and provides novel therapeutic targets for EOC. Importantly, hyperactivation of STAT3 along with upregulation of stem cell markers and epithelial mesenchymal transition (EMT) related markers have been observed in several other cancers, including glioblastoma (12) and gastric cancer cell (13). Therefore, inhibition of STAT3 may provide a broad anti-tumor strategy. It will be worth determining if delivery of miR-92a antagomir or DKK (or Wnt inhibitors) would have similar or better therapeutic effects on blocking EOC progression as anti-STAT3.
A critical unsolved question is the role of STAT3-activated CSC-like cells in spheroid formation. Spheroid formation is a complex process which involves multiple components including excellular matrix, detached tumor cells as well as large numbers of immune cells. The multicellular nature of the spheroids is thought to be attributable to adhesive molecules that mediate cell-cell and cell-secreted extracellular matrix (ECM) interactions (4,14). It was reported that the type I calcium-dependent cadherins, N- and E-cadherin on EOC cells, dominantly mediate spheroid compaction among tumor cells (15). It needs to be determined if the STAT3-mediated stem pathway increases expression of these adhesion molecules. Moreover, it is unclear that the homotypic tumor cell adhesions by these adhesion molecules on CSCs are sufficient to form stable spheroids.
In this regard, growing evidence has suggested tumor-associated macrophages (TAMs) may play an essential role in spheroid formation during the process of transcoelomic metastasis of EOC. A recent study from us have demonstrated that M2 macrophage-like TAMs were localized in the center of spheroids and can secrete a large amounts of epidermal growth factor (EGF) to activate its receptor EGFR in surrounding tumor cells. The activated EGF/EGFR signaling can upregulate VEGF-C, which in turn upregulates αMβ2 integrin on TAMs and its ligand ICAM-1 on tumor cells to form a positive feedback loop, thus promoting tumor cell proliferation, migration, adhesion, spheroid formation, and peritoneal implantation (16). The activation of EGFR/EGFR signaling and/or VEGF signaling have been implicated in the pathogenesis of many malignancies. Targeting drugs on EGFR are available on market, and for non-small cell cancer (NSCLC), EGFR agonists erlotinib has been written into guideline as the recommended first-line therapy for patients harboring EGFR mutation. Consistently, this study has proved EGFR inhibitor erlotinib drastically retarded the ovarian tumor growth with higher efficiency, highlighting the important clinical implication of targeting spheroid TAM and associated EGF/EGFR/VEGF signal pathway in the treatment of advanced EOC. It will be interesting to determine if the EGF/EGFR/VEGF signal pathway cross talks with the STAT3-mediated stem pathway, and if they synergistically contribute to the CSC-like phenotype and chemoresistance of EOC. It will be also interesting to explore if TAMs promote CSC phenotype, or conversely, CSCs preferentially associate with M2-like TAMs to facilitate spheroid formation (Figure 1).
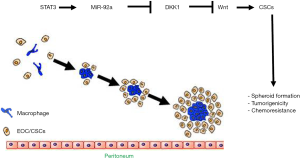
In summary, the study of Chen et al. provides important insights into the knowledge of spheroid formation and CSCs in EOC. The identification of STAT3 and its downstream molecular deregulation deep our understanding on EOC chemotherapy and provides new targets for the development of spheroid-targeted therapeutic approaches.
Acknowledgments
Funding: This work was partly supported by National Key Research and Development Program of China (2016YFC1300600), National Natural Science Foundation of China (No. 91539110), and Scientific Grants of Guangdong (No. 2015B020225002 and 2015A050502018) to WM, R01 HL109420 and R01 HL115148, and CT Stem Cell Innovation Award (Established Investigator Grant) 14-SCB-YALE-17 to WM.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Zheng Li (Department of Gynecologic Oncology, The Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.05.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Lengyel E. Ovarian cancer development and metastasis. Am J Pathol 2010;177:1053-64. [Crossref] [PubMed]
- Bookman MA, Brady MF, McGuire WP, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol 2009;27:1419-25. [Crossref] [PubMed]
- Shield K, Ackland ML, Ahmed N, et al. Multicellular spheroids in ovarian cancer metastases: Biology and pathology. Gynecol Oncol 2009;113:143-8. [Crossref] [PubMed]
- Condello S, Morgan CA, Nagdas S, et al. β-Catenin-regulated ALDH1A1 is a target in ovarian cancer spheroids. Oncogene 2015;34:2297-308. [Crossref] [PubMed]
- Kim DK, Seo EJ, Choi EJ, et al. Crucial role of HMGA1 in the self-renewal and drug resistance of ovarian cancer stem cells. Exp Mol Med 2016;48:e255 [Crossref] [PubMed]
- Liao J, Qian F, Tchabo N, et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS One 2014;9:e84941 [Crossref] [PubMed]
- Huang RY, Wong MK, Tan TZ, et al. An EMT spectrum defines an anoikis-resistant and spheroidogenic intermediate mesenchymal state that is sensitive to e-cadherin restoration by a src-kinase inhibitor, saracatinib (AZD0530). Cell Death Dis 2013;4:e915 [Crossref] [PubMed]
- Ebos JM, Lee CR, Cruz-Munoz W, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 2009;15:232-9. [Crossref] [PubMed]
- Chen MW, Yang ST, Chien MH, et al. The STAT3-miRNA-92-Wnt Signaling Pathway Regulates Spheroid Formation and Malignant Progression in Ovarian Cancer. Cancer Res 2017;77:1955-67. [Crossref] [PubMed]
- Rask K, Nilsson A, Brännström M, et al. Wnt-signalling pathway in ovarian epithelial tumours: increased expression of beta-catenin and GSK3beta. Br J Cancer 2003;89:1298-304. [Crossref] [PubMed]
- Guryanova OA, Wu Q, Cheng L, et al. Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer Cell 2011;19:498-511. [Crossref] [PubMed]
- Hajimoradi M, Mohammad Hassan Z, Ebrahimi M, et al. STAT3 is Overactivated in Gastric Cancer Stem-Like Cells. Cell J 2016;17:617-28. [PubMed]
- Graves LE, Ariztia EV, Navari JR, et al. Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Res 2004;64:7045-9. [Crossref] [PubMed]
- Puiffe ML, Le Page C, Filali-Mouhim A, et al. Characterization of ovarian cancer ascites on cell invasion, proliferation, spheroid formation, and gene expression in an in vitro model of epithelial ovarian cancer. Neoplasia 2007;9:820-9. [Crossref] [PubMed]
- Yin M, Li X, Tan S, et al. Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J Clin Invest 2016;126:4157-73. [Crossref] [PubMed]


