
Ramucirumab efficacy in first-line gastric and esophageal cancer treatment
Upper gastrointestinal cancers are highly lethal malignant diseases. Taken together gastric and esophageal (GE) cancers as a unique entity, both are estimated to be the third most incident and the second most lethal malignancies in both sexes worldwide. Despite the fact that GE cancer presents with a lower incidence in non-Asian countries with respect to Asian ones, its mortality is truly high in Occidental patients (29.3% survivors at 5 years) (1).
For advanced GE cancer patients, chemotherapy significantly improves survival in comparison with best supportive care as shown in a recent meta-analysis. However, the prognosis of advanced GE cancer patients remains poor with a median overall survival (OS) of less than 12 months after first-line treatment (1L) (2). Although the optimal 1L chemotherapy for advanced GE cancer is not determined, it is commonly based on fluoropyrimidine and platinum combinations. The addition of a third cytotoxic agent, as docetaxel, achieves a higher objective response rate (RR) and a modest survival advantage, but at the cost of an increased toxicity (3,4). Anthracycline is no longer recommended after negative results from OEO5 randomized trial, were shown (5). Five-year OS for MAGIC patients treated with platinum, fluoropyrimidine plus anthracycline was 36% and for FNCLCC/FFCD trial patients treated with platinum plus a fluoropyrimidine was 38% (6). Trastuzumab, a monoclonal antibody that inhibits the human epidermal growth factor receptor type 2 (HER-2), is the only targeted agent demonstrating an improvement in OS, when added to 1L chemotherapy for HER-2 positive gastric tumors (7). Recent ASCP and ASCO guidelines for HER-2 gastric cancer testing and clinical decision making have been published (8).
Angiogenesis is a complex molecular pathway, which is crucial for the growth and spread of multiple types of tumors and particularly in GE cancer. The vascular endothelial growth factor A (VEGF-A) is one of the most important factors in tumor angiogenesis, but other factors can also interplay in this process like different VEGF family members (VEGF-B, VEGF-C, VEGF-D, VEGF-E and VEGF-F), placental growth factor (PlGF) or the angiopoietins. VEGF-A binds receptors on the surface of the endothelial cell to drive tumor angiogenesis. Several VEGF receptors (VEGFR-1, VEGFR-2 and VEGFR-3) can interact with the soluble growth factors, but VEGFR-2 activation is an essential event in tumor angiogenesis. In addition, neuropilins are co-receptors that can enhance the activity of the VEGF receptor (9,10). Bevacizumab is a IgG1 humanized recombinant monoclonal antibody that targets the VEGF-A and blocks binding to the receptor, resulting in tumor growth inhibition. The AVAGAST phase III trial evaluated the effect of adding bevacizumab to a backbone combination of chemotherapy (cisplatin plus capecitabine) in 1L setting of advanced GE cancer. The trial did not meet the principal objective, as no difference in median OS was observed (12.1 versus 10.1 months; HR 0.87; P=0.1). The patients in the bevacizumab arm showed a statistically significant improvement in progression-free survival (PFS) (6.7 versus 5.3 months; HR 0.8; P=0.004) and in the objective RR (46% versus 37.4%; P=0.03) (11). The biomarker study conducted in this trial showed that higher serum levels of VEGF-A and lower serum levels of neuropilin-1 were associated to a trend towards an improved survival in those patients treated with bevacizumab (12). Furthermore, higher levels of circulating angiopoietin-2 were identified as an independent prognostic marker for survival but not for bevacizumab efficacy (13). Moreover, it has recently been published that bevacizumab treatment did not provide any survival benefit in loco-regional GE cancer when administered in the perioperative setting. Anastomotic leaks were more frequent in the bevacizumab arm (69 versus 30) (14). Therefore, newer combinations and strategies are needed to incorporate antiangiogenics to the 1L GE cancer armamentarium.
Although second-line (2L) chemotherapy also improves OS when compared with best supportive care for advanced GE cancer patients, the median OS remains below 6 months. Irinotecan and taxanes are the main agents with some proven efficacy in advanced GE cancer refractory to 1L treatment (15-17). Ramucirumab was the first biological agent to demonstrate a survival benefit in patients with advanced GE cancer in 2L treatment, both as a single agent or when combined with paclitaxel (18,19). Ramucirumab is a fully human IgG1 recombinant monoclonal antibody that binds to the extracellular domain of the VEGFR-2 and blocks the receptor’s activation by soluble growth factors, especially VEGF-A (20). The REGARD phase III trial evaluated the treatment with ramucirumab versus placebo in 355 patients with advanced GE cancer that had progressed to a 1L treatment. The primary endpoint of this study was met with a statistically survival benefit for the ramucirumab arm (5.2 versus 3.8 months; HR 0.77; P=0.047). There was also a significant benefit in PFS (2.1 versus 1.3 months; HR 0.48; P<0.0001) and in disease control rate (49% versus 23%). Ramucirumab treatment was associated to a higher rate of hypertension without other safety concerns (18). On the other hand, the RAINBOW phase III trial randomized 655 patients with advanced GE cancer, refractory to a 1L treatment, to receive paclitaxel or the combination of paclitaxel and ramucirumab. Overall survival, the principal endpoint of the trial, was significantly longer in the ramucirumab group (9.6 versus 7.4 months; HR 0.8, P=0.017). The patients in the ramucirumab arm also showed a significant benefit in PFS (4.4 versus 2.9 months; HR 0.64; P<0.0001) and in the objective RR (28% versus 16%; P=0.0001). The main toxicities in the ramucirumab arm were neutropenia, leucopenia, hypertension, fatigue, anemia and abdominal pain (19). Consequently, after these results, ramucirumab became a new standard treatment for advanced GE cancer refractory to a 1L treatment.
Due to the established activity of ramucirumab in the 2L setting of advanced GE cancer, some investigators have designed clinical trials to evaluate the efficacy of ramucirumab in 1L treatment of locally advanced or metastatic disease (Table 1). In the phase II trial that was published by Yoon et al., 168 patients with advanced GE adenocarcinoma were randomized to receive 1L treatment with mFOLFOX-6 plus ramucirumab or placebo. This trial was the first one to evaluate ramucirumab in 1L of GE cancer and failed to demonstrate a PFS benefit for the ramucirumab group (median of 6.4 versus 6.7 months, HR 0.98; P=0.886). Neither objective RR (45.2% versus 46.4%) nor OS (median of 11.7 versus 11.5 months) were significantly different between arms. Serious adverse events were similar in both arms, except for hypertension that was significantly more frequent in the ramucirumab group. A higher rate of premature discontinuation, not due to progressive disease, was observed in the ramucirumab arm (57% versus 31%) (21).
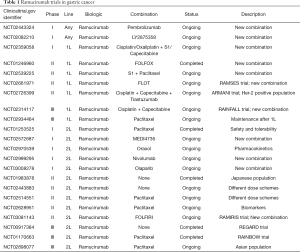
Full table
Patient selection is a key issue to success in clinical trials with cancer patients. One of the major difficulties in GE cancer is heterogeneity, which may be related to ethnicity (Caucasian versus Asian), anatomic location (esophagus versus stomach), histological type (adenocarcinoma versus squamous carcinoma), Lauren subtype (intestinal versus diffuse), molecular classification (HER-2 positive versus negative), stage (metastatic versus non-resectable locally advanced), molecular subtypes (CIN subtype shows the higher VEGF-A amplification rate), etc. It is far well known that esophageal cancer is biologically different from gastric cancer and that they may exhibit different sensitivity to chemotherapy (22,23). In fact, esophageal cancer patients were not eligible for randomization in both REGARD and RAINBOW trials (18,19). Nevertheless, in Yoon’s phase II trial, nearly half of the patients presented with tumors arising in the esophagus. In a post-hoc subgroup analysis from this trial, the tumors arising in the esophagus treated with ramucirumab showed a trend towards a worse survival compared to the tumors originated in the stomach or the GE junction (21). Mixing esophageal and gastric cancer may have influenced the negative results of the trial and it raises the question whether esophageal cancer needs specific trials apart from gastric and GE junction cancers. In addition, the high rate of discontinuation in non-progressing patients in the ramucirumab arm deserves a special attention. Most GE patients are symptomatic and deficient in nutritional status, which is not specified in this study. Considering that the safety profile reported by the authors did not differ between arms, except for hypertension, they don’t find reasons to explain the higher treatment discontinuation rate in the ramucirumab arm, in the absence of disease progression and this fact can also have an impact on outcome. Moreover, other prognostic factors like HER-2 status are not detailed in the patient population, so we do not know if some imbalance in HER-2 positivity could exist between arms. Anyway, HER-2 positive GE cancer deserves separate trials.
Despite Yoon’s phase II trial results are in line with the unproven activity of 1L bevacizumab in previous studies, future trials are exploring other combinations. In this sense, the RAINFALL trial (Table 1) is evaluating the incorporation of ramucirumab in combination with cisplatin and capecitabine, in the 1L treatment of GE cancer, excluding esophageal and HER-2 positive patients. The results of this trial will probably be communicated this year. Of course, other combinations with novel agents are ongoing, such as immunotherapy agents, pembrolizumab or nivolumab, or the combination with trastuzumab for the HER-2 positive GE cancer population. Furthermore, there is an urgent need to identify predictive biomarkers for antiangiogenic agents’ efficacy. Data from studies in hepatocellular carcinoma have shown that ramucirumab treatment increases the circulating levels of VEGF and PlGF, while decreases only transiently the levels of VEGFR-2 (24). Moreover, the tissue expressions of VEGFR-2 and HER-2, and the serum levels of VEGF-C, VEGF-D and VEGF-R-1, were analyzed in a biomarker sub-study from the REGARD trial. Unfortunately, none of the selected biomarkers were useful to predict ramucirumab efficacy in this study (25). As cancer is a dynamic reality, angiogenesis may be more relevant for GE cancer biology in 2L, after tumor progression to a 1L treatment pressure. Completed and active clinical trials with ramucirumab in GE cancer are summarized in Table 1, and biomarker studies from these trials are awaited.
To sum up, despite the negative results of the phase II trial of ramucirumab in 1L advanced gastro-esophageal cancer, future trials with ramucirumab and other targeted-oriented agents in gastro-esophageal cancer are warranted. We must focus our efforts in the development of clinical trials and translational studies evaluating combinations with novel agents that have shown activity in the preclinical setting, and in the discovery of predictive biomarkers for a better selection of patients for a specific therapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Dr. Hongcheng Zhu, MD, PhD (Department of Radiation Oncology, the First Affiliated Hospital of Nanjing Medical University, Nanjing, China)
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.05.31). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer. Available online: http://globocan.iarc.fr
- Wagner AD, Unverzagt S, Grothe W, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2010;CD004064 [PubMed]
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46. [Crossref] [PubMed]
- Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991-7. [Crossref] [PubMed]
- Cunningham D, Alderson D, Nankivell MG, et al. Toxicity, surgical complications, and short-term mortality in a randomized trial of neoadjuvant cisplatin/5FU versus epirubicin/cisplatin and capecitabine prior to resection of lower esophageal/gastroesophageal junction (GOJ) adenocarcinoma (MRC OEO5, ISRCTN01852072, CRUK 02/010). 2014 ASCO Annual Meeting. J Clin Oncol 2014; suppl 32:5s, abstr 4014.
- Ku GY. Searching for Positive Signals in Gastroesophageal Cancer. 2015 ASCO Annual Meeting. Available online: http://meetinglibrary.asco.org/content/113793?media=vm
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Bartley AN, Washington MK, Colasacco C, et al. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline From the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol 2017;35:446-64. [Crossref] [PubMed]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011;473:298-307. [Crossref] [PubMed]
- Macedo F, Ladeira K, Longatto-Filho A, et al. Gastric Cancer and Angiogenesis: Is VEGF a Useful Biomarker to Assess Progression and Remission? J Gastric Cancer 2017;17:1-10. [Crossref] [PubMed]
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. [Crossref] [PubMed]
- Van Cutsem E, de Haas S, Kang YK, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 2012;30:2119-27. [Crossref] [PubMed]
- Hacker UT, Escalona-Espinosa L, Consalvo N, et al. Evaluation of Angiopoietin-2 as a biomarker in gastric cancer: results from the randomised phase III AVAGAST trial. Br J Cancer 2016;114:855-62. [Crossref] [PubMed]
- Cunningham D, Stenning SP, Smyth EC, et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2-3 trial. Lancet Oncol 2017;18:357-70. [Crossref] [PubMed]
- Kang JH, Lee SI, Lim DH, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30:1513-8. [Crossref] [PubMed]
- Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer--a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 2011;47:2306-14. [Crossref] [PubMed]
- Ford HE, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78-86. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Javle M, Smyth EC, Chau I. Ramucirumab: successfully targeting angiogenesis in gastric cancer. Clin Cancer Res 2014;20:5875-81. [Crossref] [PubMed]
- Yoon HH, Bendell JC, Braiteh FS, et al. Ramucirumab combined with FOLFOX as front-line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: a randomized, double-blind, multicenter Phase II trial. Ann Oncol 2016;27:2196-203. [Crossref] [PubMed]
- Mercer SJ, Somers SS, Knight LA, et al. Heterogeneity of chemosensitivity of esophageal and gastric carcinoma. Anticancer Drugs 2003;14:397-403. [Crossref] [PubMed]
- Miura JT, Xiu J, Thomas J, et al. Tumor profiling of gastric and esophageal carcinoma reveal different treatment options. Cancer Biol Ther 2015;16:764-9. [Crossref] [PubMed]
- Zhu AX, Finn RS, Mulcahy M, et al. A phase II and biomarker study of ramucirumab, a human monoclonal antibody targeting the VEGF receptor-2, as first-line monotherapy in patients with advanced hepatocellular cancer. Clin Cancer Res 2013;19:6614-23. [Crossref] [PubMed]
- Fuchs CS, Tabernero J, Tomášek J, et al. Biomarker analyses in REGARD gastric/GEJ carcinoma patients treated with VEGFR2-targeted antibody ramucirumab. Br J Cancer 2016;115:974-82. [Crossref] [PubMed]


