
IQGAP1 expression in hepatocellular carcinoma predicts poor prognosis by inducing epithelial-mesenchymal transition
Introduction
Hepatocellular carcinoma (HCC) ranks as the sixth most common malignant tumor and third most frequent cancer-related cause of death worldwide (1). Chronic hepatitis infections are a predominant cause of HCC. Hepatitis B virus (HBV) infection is of a high prevalence in China. A national survey performed in 2006 showed the hepatitis B surface antigen prevalence was 7.18% in China (2). Owing to this high prevalence of HBV infection, approximately 55% of all new HCCs were diagnosed in China (3). Liver resection is perceived as a curative treatment for HCC. However, even for patients with early stage HCC, the 5-year recurrence rate was up to 50–70% after liver resection (1). Unfortunately, the detailed mechanism of postoperative recurrence is still unclear.
IQ motif containing GTPase activating proteins (IQGAPs) are multifunctional scaffold proteins characterized by isoleucine-glutamine (IQ) and GTP-activating protein (GAP) domains (4). Of the three human IQGAPs, IQ motif containing GTPase activating protein 1 (IQGAP1) is widely expressed in human tissues. A number of investigations have confirmed that overexpression of IQGAP1 is observed in many cancers, such as pancreatic cancer, breast cancer, colorectal cancer, lung cancer and others (5-8). As a scaffold protein, IQGAP1 can directly interact with Rac1 and CDC42 to regulate cell-cell adhesion and cell migration (4). Jin et al. (5) reported the proliferation, metastasis, motility and tumorigenesis ability of SW1990 human pancreatic cells could be decreased significantly by down-regulating IQGAP1 expression. In esophageal squamous cell carcinoma, Wang et al. (9) also suggested that suppressing IQGAP1 expression could greatly inhibit tumor cell growth, migration and invasion. We also observed that HCC patients with IQGAP1 overexpression had a high incidence of postoperative recurrence. However, the detailed mechanism of how IQGAP1 overexpression adversely impacts postoperative outcomes of HCC is not well established.
Increasing evidence has confirmed that the epithelial-mesenchymal transition (EMT) plays an important role in tumor progression, metastasis and drug resistance (10,11). Cells undergoing EMT show similar characteristics to cancer stem cells, which lose cell-cell adhesion structures and polarity. Recently, Dong et al. (12) confirmed that miR-124 could reverse EMT in endometrial cancer cells by attenuating the expression of IQGAP1. Therefore, we hypothesized that positive expression of IQGAP1 in HCC may increase the incidence of postoperative recurrence of HCC via inducing EMT.
In the present study, we sought to evaluate the IQGAP1 expression and its clinicopathological significance in HCC. We further identified the role of IQGAP1 in EMT in a HCC cell line.
Methods
Patients and specimens
Between 2008 and 2010, 135 HCC tissues and paired adjacent tissue samples from patients with HCC who received a liver resection in West China Hospital of Sichuan University were collected. The clinicopathological characteristics of these patients were also reviewed. This study was approved by the Ethics Review Board of West China Hospital (No.2017-62). Written informed consent was obtained from all study patients.
Follow-up and tumor recurrence
After the operation, patients were regularly monitored by blood cell tests, liver function tests, serum alpha-fetoprotein (AFP) levels, chest radiography, and visceral ultrasonography, computed tomography or magnetic resonance imaging every three months. Anti-viral treatment (entecavir or lamivudine) was administered to patients with positive preoperative hepatitis B virus-DNA (HBV-DNA) loads. Postoperative recurrence was defined as positive imaging findings compared with preoperative examinations with or without newly rising tumor marker (AFP) values or upon confirmation of recurrence by biopsy or resection (13).
Cell culture
In total, 15×104 HCC cells (HepG2/Huh7) were grown in DMEM containing 10% FBS supplemented with penicillin (100 U/mL) and streptomycin (100 mg/mL) overnight. IQGAP1 cDNA (GenBank gi57242794) was cloned into pCMV6 plasmids by a PCR-based cloning method, and the recombinant plasmid pFlag-IQGAP1 was obtained. IQGAP1-specific siRNA and negative control siRNA were purchased from Genechem (Shanghai, China). IQGAP1-specific siRNA was used as a smart pool of the following two siRNAs: 1# 5'-UUAUCGCCCAGAAACAUCUUGUUGG-3' and 2# 5'-UUCUUCAUGAGACAAGGCUUGUUCA-3'. Cell transfections of pFlag-IQGAP1 plasmids/empty plasmids and IQGAP1-specific siRNA/negative control siRNA were performed using lipofectamine 3000 following the manufacturer’s protocols. The amount of transfected plasmids was 800 ng for each well of a 6-well plate, and the IQGAP1-specific siRNA or IQGAP1-specific siRNA was used at a concentration of 800 nM in three wells of a 6-well plate. Empty plasmids or negative control siRNA were used for negative controls at the same time. The IQGAP1 overexpression or knockdown efficiency was determined by western blotting.
After being transfected with pFlag-IQGAP1 plasmids/empty plasmids or IQGAP1-specific siRNA/negative siRNA, cells were harvested 48 h later by scraping. Cells were then washed in ice-cold phosphate-buffered saline and collected with RIPA buffer (50 mM tris base, 1.0 mM EDTA, 150 mM NaCl, 0.1% SDS, 1% triton X-100, 1% sodium deoxycholate, 1 mM PMSF) (Beyotime) containing a protease inhibitor cocktail (Roche). The lysates were used to measure protein levels.
Western blot analysis
Protein samples were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto an activated-polyvinylidene difluoride membrane (Bio-Rad, United States). Immunoblotting was performed using a standard protocol. Signals were detected via chemiluminescence using an ECL kit (Millipore, United States) on a ChemiDoc XRS gel documentation system (Bio-Rad, United States). The primary antibodies used for immunoblotting included E-cadherin (Abcam ab76055), Claudin 1 (Abcam ab180158), N-cadherin (Abcam ab95440), vimentin (Abcam ab92547), MMP2 (Abcam ab97779), IQGAP1 (Abcam ab133490) and GAPDH (Abcam ab9484).
Transwell assays (migration and invasion)
Cell migration and invasion assays were performed by using transwell chambers (Chemicon) with or without matrigel according to the vendor’s instructions. After being transfected with pFlag-IQGAP1 plasmids or IQGAP1-specific siRNA or the empty plasmids or non-targeting siRNA, the cells were trypsinized and 1×105 cells were plated into the insert of the well in serum-free medium. Medium containing 10% fetal bovine serum was added to the lower chamber. After 24 h incubation at 37 °C, the cells remaining in the upper chamber or on the upper membrane were removed with a cotton swab. Cells that migrated or invaded the lower surface of the membrane were fixed with a solution containing 0.1% crystal violet and 20% methanol. Cells on the lower surface of the membrane were photographed using a TE2000 microscope (Nikon Instruments Inc., Japan) (100×), and five fields of cells were counted to estimate cell density. The mean was calculated, and the data are presented as the mean ± SD from three independent experiments performed in triplicate. The percentage of migratory cells on the condition of IQGAP1 overexpression or knockdown was calculated by comparison with the control transfection with the empty plasmids or non-targeting siRNA.
Immunohistochemistry
The paraffin-embedded hepatic specimens were cut into 5 µm thick tissue sections, and slides were prepared using standard techniques. The IQGAP1 antibody was diluted 1:500 (sc-376021, Santa Cruz). Tissue slices were visualized using DAB chromogen substrate and counterstained with hematoxylin. The immunohistochemistry outcomes were assessed by two independent pathologists using light microscopy. The estimated percentage of staining was determined by calculating average staining cells of five views (100 cells per view at 400-fold magnification). The percentage of positive staining was scored 0–4. No positive staining was defined as 0, less than 10% positive staining was scored as 1, 10–50% positive staining was scored as 2, 50–75% positive staining was scored as 3, and greater than 75% positive staining was scored as 4. Staining intensity was defined as 4 levels as follows: 0, negative; 1, weak; 2, moderate; and 3, strong. The immunoreactivity score for each slice was calculated by immunostaining intensity multiplied by the proportion of positive cells (ranged from 0 to 12). The immunoreactivity scores of 0-3 were accepted as negative staining, and scores ≥4 were accepted as positive staining.
Statistical analyses
All statistical analyses were performed by SPSS 22.0 for Windows. All continuous variables are presented as the mean ± SD and were compared using one-way analysis of variance. Categorical variables were compared using the X2 test or Fisher’s exact test. The independent risk factors for recurrence-free survival (RFS) and overall survival (OS) were identified by Cox regression. The Kaplan-Meier method was used to compare the postoperative RFS and OS for different groups. The differences in the RFS and OS curves were compared using a log-rank test. A P value of less than 0.05 was considered statistically significant.
Results
Demographic data of patients
A total of 135 patients were included in this study, including 125 males and 10 females. The mean age was 50.56±12.26 years. The mean tumor size was 3.33±1.02 cm. Eleven patients had multiple tumors. Microvascular invasion (MVI) was detected in 44 patients. The AFP level of 52 patients was greater than 400 ng/mL. Positive HBV-DNA was observed in 79 patients. In 135 HCC samples, positive IQGAP1 expression was detected in 78 patients (57.78%), which was significantly higher than in the adjacent samples (n=11, 8.15%; P<0.001). With a mean of 56.86±21.10 months for the follow-up period, 81 patients suffered from recurrence, whereas 50 patients died. The 1-, 3-, and 5-year RFS rates were 79.3%, 59.3%, and 45.9%, respectively, whereas the 1-, 3-, and 5-year OS rates were 98.5%, 79.0%, and 64.8%, respectively (Figure 1A,B).
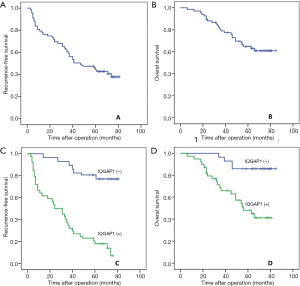
Comparison of clinicopathological data of patients with positive IQGAP1 staining and negative IQGAP1 expression
As shown in Table 1, more patients with MVI and multiple tumors were observed among patients with positive IQGAP1 expression in HCC tissues. There was no significant difference with respect to age, gender, tumor difference, high AFP level, total bilirubin, albumin or tumor size between the two groups.

Full table
Risk factor analysis for postoperative recurrence
In the univariate analysis, positive IQGAP1 expression, high preoperative AFP level, MVI, and multiple tumors showed potential prognostic power. However, only positive IQGAP1 expression and high preoperative AFP level were independent risk factors for postoperative recurrence in the multivariate analysis (Table 2).

Full table
Risk factors analysis for postoperative OS
As listed in Table 3, positive IQGAP1 expression, high preoperative AFP level, low preoperative albumin level, MVI, and multiple tumors were potential risk factors in the univariate analysis. In the multivariate analysis, IQGAP1 expression, low preoperative albumin level, and MVI were associated with low OS after liver resection.

Full table
Comparison of RFS and OS between patients with positive versus negative IQGAP1 expression in HCC tissues
As presented in Figure 1, the 1-, 3-, and 5-year RFS rates for patients with positive IQGAP1 expression were significantly lower than those with negative expression (64.1%, 37.2% and 17.9% versus 100%, 93.0% and 80.7%; P<0.001; Figure 1C). The 1-, 3-, and 5-year OS rates were 97.4%, 68.5% and 48.1%, respectively, for patients with positive IQGAP1 expression in HCC tissues, and 100%, 96.5% and 86.0%, respectively, for patients with negative IQGAP1 expression in HCC tissues (P<0.001; Figure 1D).
IQGAP1 expression is associated with an EMT phenotype
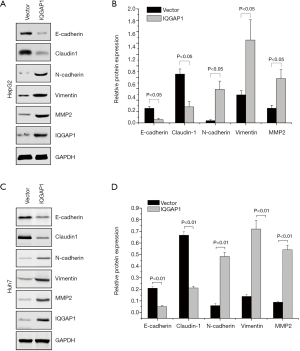
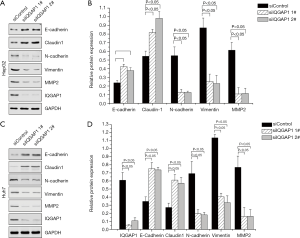
As shown in Figures 2 and 3, we assessed the EMT-associated proteins of HCC cell lines (HepG2 and Huh7) after being transfected with pFlag-IQGAP1 plasmids/empty plasmids or IQGAP1-specific siRNA/negative siRNA. When IQGAP1 was overexpressed in HepG2/Huh7 cells by transfection with pFlag-IQGAP1 plasmids, the epithelial markers E-cadherin and Claudin 1 were down regulated, whereas the mesenchymal markers N-cadherin, vimentin and MMP2 were up regulated (Figure 2). On the contrary, following silencing of IQGAP1 in HepG2/Huh7 cells by IQGAP1-specific siRNA, high expressions of E-cadherin and Claudin 1 and low expressions of N-cadherin, Vimentin and MMP2 were observed (Figure 3). These data suggested IQGAP1 was associated with EMT.
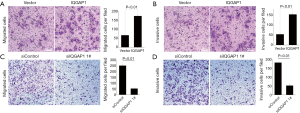
Transwell assay
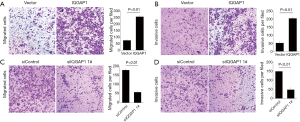
Transwell invasion assays indicated that, following the 48 h incubation, when IQGAP1 was overexpressed by pFlag-IQGAP1 plasmids, more HCC cells passed through the transwell membranes compared to controls (Figures 4,5). On the contrary, less HCC cells passed the through the transwell membranes when IQGAP1 was silenced (Figures 4,5). These data suggested overexpression of IQGAP1 could increase the invasive and migration ability of HCC cells, whereas, silencing of IQGAP1 in the HCC cells could reduce the invasive and migration ability.
Discussion
IQGAP1 is a multifunctional scaffold protein widely expressed in human tissues. A number of studies have confirmed that IQGAP1 expression was associated with poor outcomes in breast cancer, pancreatic cancer, colorectal cancer, non-small cell lung cancer and other cancers (5,7,8). Wang et al. (14) confirmed that the IQGAP1 expression level in pancreatic cancer tissues was significantly higher than in adjacent tissues. The level of IQGAP1 expression was associated with grades of cancer differentiation. Hayashi and his colleague’s study indicated that overexpression of IQGAP1 in colorectal cancer correlates with a poor prognosis via improving the ability of cancer cell invasion (7). Our study suggested that positive expression of IQGAP1 in HCC contributed to a high incidence of postoperative recurrence mainly via inducing EMT.
Our study suggested that the IQGAP1 positive expression group had more patients with MVI. Previous investigations have confirmed that MVI is a risk factor for postoperative recurrence for HCC following liver resection (15-17). Even for patients with HCC within Milan criteria who were treated with liver transplantation, MVI also increased the incidence of postoperative recurrence and shortened the recipient’s OS (18). HCC patients with MVI may have latent micro-metastasis before operation. Our experimental study suggested that with overexpression of IQGAP1 in HCC cell lines, the epithelial markers E-cadherin and Claudin 1 were down regulated, whereas the mesenchymal markers N-cadherin, vimentin and MMP2 were up regulated. These results indicated the overexpression of IQGAP1 could induce EMT. A number of investigations have confirmed that the EMT phenomenon was associated with a very aggressive tumor behavior. Our study also confirmed the invasive and migration ability of HCC cells, which was detected by the transwell assay, could also be enhanced by overexpression of IQGAP1 in HCC cells. During the follow-up period, HCC patients with positive IQGAP1 expression also suffered from higher incidences of postoperative recurrence and poor long-term survival.
Similar to MVI, more patients with positive IQGAP1 expression were observed in patients with multiple tumors. Multiple HCCs may be either intrahepatic metastases from a primary HCC or multicentric in origin. Wu et al. (19) confirmed that multiple tumors were an independent risk factor for both early and late recurrence for HCC patients after liver resection. Our study suggested that more patients with multiple tumors had positive IQGAP1 expression. We think this phenomenon may be due to some multiple HCCs being intrahepatic metastases.
High preoperative AFP was an independent risk factor for postoperative recurrence in the current study. Previous studies also suggested that high preoperative AFP contributed to poor prognosis of patients with HCC after liver resection or liver transplantation (13,20). Graham et al. (21) even suggested that liver transplantation should be offered to patients with HCC within Milan criteria and a positive serum AFP rather than liver resection.
It was interesting that low albumin was a risk factor associated with postoperative mortality, even if low albumin was not related to postoperative recurrence. Albumin is a marker used to assess a patient’s liver function. Patients with low albumin had poor liver function. Recently, some investigations also confirmed some prognostic markers including albumin could predict the prognosis of HCC after liver resection (22,23).
IQGAP1 was able to promote tumor proliferation and metastasis in some other tumors. Jin et al. (5) suggested IQGAP1 could improve the metastasis of pancreatic cancer through activating the Cdc42/Rac1 pathway. Ma et al. (24) reported that the overexpression of IQGAP1 could improve the cell proliferation of multiple myeloma by regulation of the MAP kinase (ERK1/2) pathway. Meng et al. (6) confirmed that IQGAP1 may directly interact with ERα and serve as a signal integrator, regulating the activity of ERα transcription, cell proliferation and cell invasion during the progression of thyroid cancer. Different from previous studies, our study suggested IQGAP1 might improve the proliferation and invasion of HCC via inducing EMT. However, how IQGAP1 induces EMT is unknown and deserves further study.
In conclusion, our study suggests that positive expression of IQGAP1 is associated with a high incidence of postoperative recurrence and poor OS for patients with HCC after liver resection. IQGAP1 could induce EMT in HCC.
Acknowledgments
Funding: This study was funded by Scientific and Technological Support Project of Sichuan Province (2016SZ0025 and 2015SZ0049).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.05.40). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was the Ethics Review Board of West China Hospital (No. 2017-62) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- European Association For The Study Of The Liver. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Cui Y, Jia J. Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol 2013;28:7-10. [Crossref] [PubMed]
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [Crossref] [PubMed]
- Kuroda S, Fukata M, Nakagawa M, et al. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin- mediated cell-cell adhesion. Science 1998;281:832-35. [Crossref] [PubMed]
- Jin Y, Lv X, Zhou J, et al. Potential involvement of IQGAP1 in proliferation and metastasis of human pancreatic cancer. Front Biosci (Landmark Ed) 2016;21:1076-83. [Crossref] [PubMed]
- Meng D, Wu W, Li Z, et al. IQGAP1 modulates the proliferation and invasion of thyroid cancer cells in response to estrogen. Int J Mol Med 2015;36:588-94. [PubMed]
- Hayashi H, Nabeshima K, Aoki M, et al. Overexpression of IQGAP1 in advanced colorectal cancer correlates with poor prognosis-critical role in tumor invasion. Int J Cancer 2010;126:2563-74. [PubMed]
- Zhao H, Xie C, Lin X, et al. Coexpression of IQ-domain GTPase-activating protein 1 (IQGAP1) and Dishevelled (Dvl) is correlated with poor prognosis in non-small cell lung cancer. PLoS One 2014;9:e113713 [Crossref] [PubMed]
- Wang XX, Wang K, Li XZ, et al. Targeted knockdown of IQGAP1 inhibits the progression of esophageal squamous cell carcinoma in vitro and in vivo. PLoS One 2014;9:e96501 [Crossref] [PubMed]
- Chen HA, Kuo TC, Tseng CF, et al. Angiopoietin-like protein 1 antagonizes MET receptor activity to repress sorafenib resistance and cancer stemness in hepatocellular carcinoma. Hepatology 2016;64:1637-51. [Crossref] [PubMed]
- Matsumoto R, Tsuda M, Yoshida K, et al. Aldo-keto reductase 1C1 induced by interleukin-1beta mediates the invasive potential and drug resistance of metastatic bladder cancer cells. Sci Rep 2016;6:34625. [Crossref] [PubMed]
- Dong P, Ihira K, Xiong Y, et al. Reactivation of epigenetically silenced miR-124 reverses the epithelial-to-mesenchymal transition and inhibits invasion in endometrial cancer cells via the direct repression of IQGAP1 expression. Oncotarget 2016;7:20260-70. [PubMed]
- Zheng SS, Xu X, Wu J, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 2008;85:1726-32. [Crossref] [PubMed]
- Wang XX, Li XZ, Zhai LQ, et al. Overexpression of IQGAP1 in human pancreatic cancer. Hepatobiliary Pancreat Dis Int 2013;12:540-5. [Crossref] [PubMed]
- Zhao H, Chen C, Fu X, et al. Prognostic value of a novel risk classification of microvascular invasion in patients with hepatocellular carcinoma after resection. Oncotarget 2017;8:5474-86. [PubMed]
- Lei Z, Li J, Wu D, et al. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg 2016;151:356-63. [Crossref] [PubMed]
- Feng LH, Dong H, Lau WY, et al. Novel microvascular invasion-based prognostic nomograms to predict survival outcomes in patients after R0 resection for hepatocellular carcinoma. J Cancer Res Clin Oncol 2017;143:293-303. [Crossref] [PubMed]
- Kim JM, Kwon CH, Joh JW, et al. Effectiveness of locoregional therapy before living donor liver transplantation in patients with hepatocellular carcinoma who meet the Milan criteria. Transplant Proc 2012;44:403-8. [Crossref] [PubMed]
- Wu JC, Huang YH, Chau GY, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol 2009;51:890-7. [Crossref] [PubMed]
- Goh BK, Kam JH, Lee SY, et al. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and prognostic nutrition index as preoperative predictors of early mortality after liver resection for huge (>/=10 cm) hepatocellular carcinoma. J Surg Oncol 2016;113:621-7. [Crossref] [PubMed]
- Graham JA, Melancon JK, Shetty K, et al. Liver transplantation should be offered to patients with small solitary hepatocellular carcinoma and a positive serum alpha fetoprotein rather than resection. Am J Surg 2013;205:374-80. [Crossref] [PubMed]
- Shim JH, Jun MJ, Han S, et al. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Ann Surg 2015;261:939-46. [Crossref] [PubMed]
- Wang YY, Zhong JH, Su ZY, et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg 2016;103:725-34. [Crossref] [PubMed]
- Ma Y, Jin Z, Huang J, et al. IQGAP1 plays an important role in the cell proliferation of multiple myeloma via the MAP kinase (ERK) pathway. Oncol Rep 2013;30:3032-8. [PubMed]


