
Treatment outcome and prognostic factor in fit elderly patients with multiple myeloma received frontline induction of bortezomib based regimen (PAD or VCD)
Introduction
Multiple myeloma (MM) is an incurable hematological malignancy originating from plasma cell. With conventional chemotherapy, such as melphalan and prednisone (MP), vincristine, adriamycin and dexamethasone (VAD), the overall response rate (ORR) and complete remission (CR) rate was approximately 60% and less than 5%, respectively, with a median overall survival (OS) time of 2 to 3 years (1). Throughout the last decade, the advent of proteasome inhibitors, such as bortezomib, lead to a revolution in MM treatment (2,3). A significantly higher CR rate (30–40%) could be achieved by using bortezomib based regimens as the frontline treatment, which could be translated to a significant improved OS (2006–2010 vs. 2001–2005: 5 vs. 3.2 years) (4-6). Hence, chemotherapy of bortezomib-based combination with two or three drugs, are recommended as standard frontline induction therapy in both transplant-eligible and -ineligible patients with MM.
Newly diagnosed MM patients aged over 65 years are generally defined as elderly MM; and not considered to be the transplant candidates. For these patients, the regimen recommended as the first line treatment (category 1) in most western guidelines included: melphalan/prednisone/bortezomib (MPB), melphalan/prednisone/thalidomide (MPT), lenalidomide/bortezomib/dexamethasone (RVD) or lenalidomide/dexamethasone (RD), on the other hand more aggressive cytotoxic agents, especially doxorubicin, which is generally suggested in younger patients, is still controversial in this group of the patients. However, due to the problem of availability of melphalan in china and registration approval of lenalidomide in frontline, we generally used bortezomib/doxorubicin/dexamethasone (PAD) or bortezomib/cyclophosphamide/dexamethasone (VCD) as the first line therapy for transplant-ineligible patients. And of note, there is no head to head study comparing PAD and VCD in elderly patients with MM.
Considering elderly patients being highly heterogeneous, the International Myeloma Working Group (IMWG) suggested the use of geriatric assessment (GA) which divides elderly patients into 3 groups: fit, intermediate-fitness, and frail. Tailored therapy was also recommended to each group (7). Precise analysis of the effects and safety of the regimens should be performed in each specific sub-group. Also, not many reports focus on the subset of the fit elderly patients with MM. Therefore, we consecutively included 64 fit elderly patients with newly diagnosed multiple myeloma (NDMM) who received PAD or VCD as induction therapy followed by a maintenance of thalidomide, to analyze its efficacy, adverse effects and prognostic factors in Chinese patients.
Methods
Patients
From May 2009 to May 2014, 64 elderly patients with NDMM aged from 65 to 75 years were included. Since we could not collect the complete data of activities of daily living (ADL) and instrumental activities of daily living (IADL) of the patients, we modified the conditions of fitness, which were defined as ‘age from 65 to 75 years’ and ‘Charlson index [0–1]’. They received either PAD or VCD induction therapy in Ruijin Hospital Affiliated to Shanghai Jiao Tong University, School of Medicine. The study was approved by the ethic committee of Ruijin Hospital and performed according to the Declaration of Helsinki. Informed consents were obtained from all the patients.
Evaluation of the disease and adverse events
MM was diagnosed according to standard criteria of IMWG. All the 64 patients were with symptomatic MM and measurable disease (non-secretary MM was excluded); all of them were full dose chemotherapy eligible (severe heart, lung or liver disease at baseline was excluded). The response criteria was according to the IMWG uniform response criteria for MM, including CR, very good partial response (VGPR), partial response (PR), stable disease (SD) and progressive disease (PD) (8). Adverse events were assessed and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE, version 4.0).
Treatment regimens
PAD or VCD regimen was given to the patients according to the decision of the physician. Bortezomib was administrated 1.3 mg/day intravenously on days 1, 4, 8, 11 in a 28-day cycle. Doxorubicin was given 9 mg/day intravenously on days 1–4; cyclophosphamide was given 300 mg/day intravenously on days 1, 4, 8, 11; dexamethasone was administrated 40 mg/day intravenously on days 1–4. All the patients received maintenance thalidomide 50–100 mg/day orally, if tolerated, continuing until disease progression. Antithrombotic agents were not routinely used. Antiviral prophylaxis (acyclovir 400 mg/day orally) has been given mandatory throughout introduction therapy since the year 2010.
Statistical analysis
Fisher’s exact test was used to compare the differences in CR rates. One way Anova test was used to compare baseline characteristics in two groups. Progressive free survival (PFS) was defined as from start of treatment to disease progression, relapse, or any cause of death. OS was calculated from the diagnosis to any cause of death. Kaplan-Meier and hazard ratio analysis was used to calculate and compare the survival outcomes. Cox model was used for the multivariate analysis of associations of survival and potential prognostic factors. A limited backward selection procedure was used to exclude redundant varieties. All analyses were performed with the Statistical Software Package for the Social Sciences (SPSS version 19.0 for Windows; SPSS Inc., Chicago, IL, USA).
Results
Patients
Sixty-four patients were included, among which, 39 (61%) were male and 25 (39%) were female. The median age at diagnosis in this study was 67 years (range, 65–75 years). Of all the patients, 44 (68.75%) cases were within international staging system (ISS) stage II, 20 (30.25%) cases were within ISS stage III. There was no significant difference in patient characteristics between PAD and VCD group. The detailed baseline characteristics were listed in Table 1.

Full table
Treatment outcomes
Eight cycles of PAD and VCD was original scheduled for the patients, however, a sizable portion of patients could not complete the whole regimen according to their tolerability or personal will (mostly economic reason). In both two groups, the median cycles of induction treatment were four.
After the induction therapy, the ORR/PR of all the 64 patients was 93.7% (60/64), CR rate was 32.8% (21/64). There was no significant difference between PAD and VCD group, regarding ORR (90.6% vs. 96.8%, respectively) and CR rate (37% vs. 28%, respectively) (Table 2).
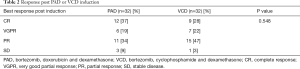
Full table
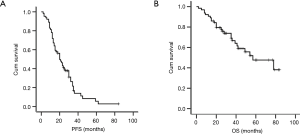
At the time of analysis (May 31, 2016), the median follow up time was 28 months (range, 8–79 months), the median PFS and OS were 21.6 and 56.8 months for the entire group respectively (Figure 1).
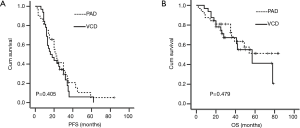
In the PAD group, 81% (26/32) patients progressed or relapsed, and 40% (13/32) patients died. In the VCD group, 84% (27/32) patients progressed or relapsed, and 43% (14/32) patients died. There was no difference on the survival between the two groups. Two-year PFS rates were 41.4% and 40.6% for PAD and VCD group, respectively (P=0.405, Figure 2A), and five-year OS rates were 51.2% and 41.2%, respectively PAD and VCD group, respectively (P=0.479, Figure 2B).
Adverse events
Treatment related death happened in one patient in PAD group, who died from pneumonia in cycle 2. Other serious adverse events included two pneumonias and one heart failure in PAD group, and two pneumonias in VCD group.
In PAD group, the scheduled regimen was postponed in 9 patients’ treatments for AE. The dose of doxorubicin was reduced to 75% in three patients, and the dose of bortezomib was reduced to 1.0 mg/m2 in five patients. In VCD group, treatment was postponed in five patients for AE, the dose of bortezomib was reduced to 1.0 mg/m2 in seven cases.
During the period of induction therapy, hematologic toxicities (grade 3 to 4) included neutropenia (15.6–21.8%), anemia (15.6%) and thrombocytopenia (12.5–18.7%). The most frequent non-hematologic toxicities included infection, fatigue, constipation, diarrhea, and herpes zoster, as list in Table 3, cardiac toxicity was rare, which was observed in two patients (one congestive heart failure and one atrial fibrillation) in the PAD group. Peripheral neuropathy (PN) was reported in 18 (56.2%; PAD) and 20 (62.5%; VCD) patients, with 5 (15.6%; PAD) and 7 (21.8%; VCD) for grade ≥2. There was no difference between the PAD group and VCD group, regarding both hematological and non-hematological toxicities (Table 3).
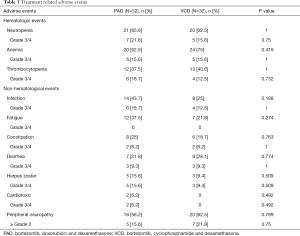
Full table
Prognostic analysis
In the univariate Cox analysis, we included several parameters as potential prognostic factors, such as gender, lactate dehydrogenase (LDH), albumin (ALB), ISS, Durie-Salmon (DS), CR, and serum β2 microglobulin (β2-m). It was proved that only LDH level (elevated vs. normal) and ALB level (<35 g/L vs. normal) were associated with poor OS (HR=2.966, 95% CI, 1.287–6.833, P=0.011 and HR=3.212, 95% CI, 1.106–9.332, P=0.032).
In the multivariate analysis for OS, the risk of death increased for the patients with high LDH (OR=2.715, 95% CI, 1.184–6.223, P=0.018). And ALB <35 g/L was also an independent factor associated with short OS (OR=2.980, 95% CI, 1.021–8.699, P=0.046).
Other factors, such as DS, CR and β2-MG were not associated with the OS (Table 4).

Full table
Discussion
Recently, with the use of novel agents, such as bortezomib, thalidomide and lenalidomide, better outcomes have been achieved in elderly patients with MM. Generally, regimens such as MPT, MPV, MPR and Rd were recommended as the frontline induction therapy to elderly patients, however, for some specific reasons, the combination of PAD or VCD which was initially designed for younger patients was more frequently used in China, especially for elderly patients aged under 75 years. Until now, very few clinical trials were compared head to head with PAD and VCD regimen in MM. Recently, a phase III trial data showed that VCD was favorable to PAD as induction therapy in transplant-eligible patients. But in this study, the dosage of dexamethasone was higher in VCD group than that in the PAD group (9). Some phase II trials showed that PAD or VCD induction therapies in all cohorts (both young and elderly) can dramatically improve the outcome in MM, with an ORR (at least PR) of 88–90% and a CR rate of 40% (5,10,11). But no study comparing those two regimens has been performed for transplant-ineligible patients. It was the first time we focused on the elderly patients with MM in our hospital who received either PAD or VCD as frontline treatment.
In elderly patients with either MPB or MPT induction therapy, the ORR could reach 71–80%, including 20–30% CRJeny (12-14). In our study, with PAD or VCD, the ORR (at least PR) of all the 64 elderly myeloma patients was 93.7%, including 32.8% CR. The result was better than that of the literatures. The younger age (mean age was 67 years) and better physical condition in our study might be the reason. In 2011, a report from the European Myeloma Network (EMN) points out that personalized therapy should be made in elderly MM according to the patient’s age and vulnerability (15). In 2015, IMWG proposed GA score to evaluate patients’ fitness (7). It is necessary to evaluate the effectiveness of different chemotherapy and dosage in each subset in elderly patients. In this study, we only focus on the fit elderly patients. Since this study is a retrospective one, it is difficult to collect all the required data for GA; a simple definition of fit was used. We believe that it is reasonable to perform a prospective, randomized clinical trial to further confirm the results of the studied cohort. In the future study, GA score should be used to precisely define the fit sub-group.
No significant difference was observed between the PAD and VCD group’s ORR and CR (90.6% versus 96.8% and 37% versus 28%, respectively). Unlike younger patients, very few patients in our group could complete the whole eight scheduled cycles of treatment, due mostly to the tolerability. The median treatment cycles were four, reflecting that in fit elderly patients, although could receive intense triplet agents, a shortened induction cycle was still suggested. A way to improve tolerability which might be considered; Reeder et al modified the regimen to a once-weekly schedule of bortezomib. Even after modification, results reached an ORR of 93% and a VGPR of 60%. In addition, only a few dose reductions or treatment interruption were required in the modified schedule (11).
At present, prognostic factors of MM patients include both host factors and disease factors, such as gender, ALB, LDH, cytogenetics, DS stage and ISS. ISS stage which was based on serum 2-microglobulin and ALB, could classify the patients into three risk groups (I/II/III), with median survivals of 62, 44 and 29 months, respectively (16). In our study, ISS appears to be less helpful in predicting the OS, which was also proved by several other reports (17,18). It was suggested that with bortezomib based induction, the poor impact of ISS III on the survival rate might be abrogated; however, further prospective clinical trials are warranted. Of note, in our univariate and multivariate Cox analysis, low ALB (one parameter of the ISS) became an independent factor predicting poor survival; concurrent with a recent study led by Chen et al. who also reported that initial low serum ALB increased mortality risk in MM (P=0.029) (19). In our study, another important finding revealed that elevated LDH was strongly associated with short survival during both univariate and multivariate analysis. The elevated serum LDH level commonly reflected an aggressive disease, a high proliferation rate, and a high tumor load. Even in the era of the novel agent use, high LDH remain an independent prognostic factor for poor OS (20). In the year 2015, IMWG has remodified the ISS for MM. In the revised international staging system (R-ISS), LDH was combined as a new parameter to ISS (21).
As for the adverse events, PAD and VCD groups showed a similar safety profile. The most common non-hematologic toxicities included: fatigue, infection, constipation, diarrhea, nausea and herpes zoster; which were comparable to the previous reports. We reported a lower incidence of herpes zoster (9.4–15.6%), as compared with the literatures (13–22%) (22,23), which might be attributed to the routine anti-viral therapy after year 2010 in our hospital. PN is another frequent adverse event of bortezomib. Peng et al. performed a meta-analysis of 34 clinical trials, which reported a PN incidence in all grades and high grade of 33.9% and 8.1%, respectively (24). In our cohort, the incidence rate was little higher, 56.2–62.5% for all grades and 15.6–21.8% for grade ≥2. Furthermore, in this study, about 20% dose adjustment and skipping was associated with PN. The difference in ethnic and genetic background or the frailty of elderly patients might be the reason. Even during later treatments where the dosage of bortezomib was reduced in the patients with PN; the curative effect was not affected. Interestingly, some trials affirmed PN being associated with an improved survival. The higher response rate and a longer time to progression were observed in PN experienced patients when compared with those who did not (25,26). In order to avoid adverse events such as PN in the future, weekly or subcutaneous administration of bortezomib will perhaps start to see more common use. This study will surely be worth a try in our center in the future.
In conclusion, bortezomib-based triplet regimens were tolerable and efficacious in fit elderly patients with NDMM. There was no substantial difference between PAD group and VCD group for either outcomes or safety. Low ALB and high LDH were independent prognostic factors in elderly patients, even in the era of the novel agents.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (grant No. 81302038 and 81370653)
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.06.17). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethic Committee of Ruijin Hospital (2014 NO 59) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- . Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6,633 patients from 27 randomized trials. Myeloma Trialists' Collaborative Group. J Clin Oncol 1998;16:3832-42. [Crossref] [PubMed]
- Kumar S, Flinn I, Richardson PG, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood 2012;119:4375-82. [Crossref] [PubMed]
- Chanan-Khan AA, Giralt S. Importance of achieving a complete response in multiple myeloma, and the impact of novel agents. J Clin Oncol 2010;28:2612-24. [Crossref] [PubMed]
- Popat R, Oakervee HE, Hallam S, et al. Bortezomib, doxorubicin and dexamethasone (PAD) front-line treatment of multiple myeloma: updated results after long-term follow-up. Br J Haematol 2008;141:512-6. [Crossref] [PubMed]
- Reeder CB, Reece DE, Kukreti V, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia 2009;23:1337-41. [Crossref] [PubMed]
- Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 2014;28:1122-8. [Crossref] [PubMed]
- Palumbo A, Bringhen S, Mateos MV, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood 2015;125:2068-74. [Crossref] [PubMed]
- Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia 2006;20:1467-73. [Crossref] [PubMed]
- Mai EK, Bertsch U, Durig J, et al. Phase III trial of bortezomib, cyclophosphamide and dexamethasone (VCD) versus bortezomib, doxorubicin and dexamethasone (PAd) in newly diagnosed myeloma. Leukemia 2015;29:1721-9. [Crossref] [PubMed]
- Palumbo A, Gay F, Falco P, et al. Bortezomib as induction before autologous transplantation, followed by lenalidomide as consolidation-maintenance in untreated multiple myeloma patients. J Clin Oncol 2010;28:800-7. [Crossref] [PubMed]
- Reeder CB, Reece DE, Kukreti V, et al. Once- versus twice-weekly bortezomib induction therapy with CyBorD in newly diagnosed multiple myeloma. Blood 2010;115:3416-7. [Crossref] [PubMed]
- Mateos MV, Oriol A, Martinez-Lopez J, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol 2010;11:934-41. [Crossref] [PubMed]
- San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med 2008;359:906-17. [Crossref] [PubMed]
- Palumbo A, Bringhen S, Liberati AM, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood 2008;112:3107-14. [Crossref] [PubMed]
- Palumbo A, Bringhen S, Ludwig H, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN). Blood 2011;118:4519-29. [Crossref] [PubMed]
- Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol 2005;23:3412-20. [Crossref] [PubMed]
- Kuroda J, Shimura Y, Ohta K, et al. Limited value of the international staging system for predicting long-term outcome of transplant-ineligible, newly diagnosed, symptomatic multiple myeloma in the era of novel agents. Int J Hematol 2014;99:441-9. [Crossref] [PubMed]
- Maltezas D, Dimopoulos MA, Katodritou I, et al. Re-evaluation of prognostic markers including staging, serum free light chains or their ratio and serum lactate dehydrogenase in multiple myeloma patients receiving novel agents. Hematol Oncol 2013;31:96-102. [Crossref] [PubMed]
- Chen JH, Hsu SN, Huang TC, et al. Prognostic Significance of Initial Serum Albumin and 24 Hour Daily Protein Excretion before Treatment in Multiple Myeloma. PLoS One 2015;10:e0128905 [Crossref] [PubMed]
- Chim CS, Sim J, Tam S, et al. LDH is an adverse prognostic factor independent of ISS in transplant-eligible myeloma patients receiving bortezomib-based induction regimens. Eur J Haematol 2015;94:330-5. [Crossref] [PubMed]
- Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol 2015;33:2863-9. [Crossref] [PubMed]
- Chanan-Khan A, Sonneveld P, Schuster MW, et al. Analysis of herpes zoster events among bortezomib-treated patients in the phase III APEX study. J Clin Oncol 2008;26:4784-90. [Crossref] [PubMed]
- Kim SJ, Kim K, Kim BS, et al. Bortezomib and the increased incidence of herpes zoster in patients with multiple myeloma. Clin Lymphoma Myeloma 2008;8:237-40. [Crossref] [PubMed]
- Peng L, Ye X, Zhou Y, et al. Meta-analysis of incidence and risk of peripheral neuropathy associated with intravenous bortezomib. Support Care Cancer 2015;23:2813-24. [Crossref] [PubMed]
- Dimopoulos MA, Mateos MV, Richardson PG, et al. Risk factors for, and reversibility of, peripheral neuropathy associated with bortezomib-melphalan-prednisone in newly diagnosed patients with multiple myeloma: subanalysis of the phase 3 VISTA study. Eur J Haematol 2011;86:23-31. [Crossref] [PubMed]
- Richardson PG, Briemberg H, Jagannath S, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol 2006;24:3113-20. [Crossref] [PubMed]


