
The importance of novel molecular biomarker of early stage lung adenocarcinoma
Lung cancer is one of the most frequent human cancers and the leading cause of cancer related death worldwide compared with other solid tumors (1). Surgical resection is the standard treatment of patients with early stage non-small cell lung cancer (NSCLC). Despite curative intent surgical resection, tumor recurrence and metastasis remain the primary causes of cancer-related death (1). With the results of the National Lung Screening Trial, the detection rate and the opportunity of curative treatment for early-stage lung cancer is expected to increase. Based on this study, lung cancer mortality can be reduced if tumors are diagnosed in early stage (2). The overall prognostic outcome of early stage lung adenocarcinoma (ADC), which is the major pathological subtype of NSCLC, is favorable compared to advanced stage lung ADCs and other histological subtypes. However, up to 17% of these patients will eventually relapse within 5 years from initial surgery (3). Most of the reasons of the deaths are due to distant recurrence after surgical resection (3). This dilemma suggests that early stage lung cancer patients may have occult metastasis or circulating tumor cells at the time of resection. These patients may benefit from an adjuvant treatment like patients with advanced disease at the time of surgery. Adjuvant chemotherapy has been suggested as the standard of care for stage II and III patients based on results of several randomized control studies and most recently summarization in the Lung Adjuvant Cisplatin Evaluation meta-analysis of multiple cisplatin based trials (4,5). In contrast, the benefit of adjuvant chemotherapy in stage I lung ADC remains controversial (5,6). Under the circumstance, surgery alone was the only standard treatment option for stage I ADC patients. Current National Comprehensive Cancer Network (NCCN) guidelines approve adjuvant chemotherapy for patients with stage IB lung ADC with high risk features which is the basis of tumor size following results from the CALGB 9633 trial (6). Recent findings of a new lung cancer classification suggest that only tumor size may not be sufficient when deciding lung cancer aggressiveness in early stage lung ADC (3). The CALGB 30506 trial was initiated in lung cancer to clinically examined the lung molecular prognostic signature (7). The perceptions of this trial are to (I) determine the potential survival benefit of adjuvant treatment in stage I NSCLC; (II) determine the potential survival benefit of adjuvant treatment in predicted high risk stage I NSCLC patients; and (III) determine the survival difference between the predicted high and low risk groups who are not given adjuvant chemotherapy. Even if adjuvant chemotherapy is found to be beneficial in stage I patients predicted to be high risk, it will be important to establish that the patients who benefit could not have been identified based on tumor size and other standard clinicopathological risk factors. Therefore, struggles have been dedicated to the development of better prognostic biomarkers to classify patients at risk of early recurrence following curative-intent surgical resection and those who have a high risk of death following recurrence and high risk for the occult and micro metastasis, and the selection of those patients who might benefit from multimodality adjuvant treatment.
Genomics studies may help in identifying molecular subtypes of stage I lung ADC associated with poor prognosis. Lately, many clinical and molecular factors have been evaluated as potential biomarkers in several cancers. For instance, tumor molecular signatures have demonstrated high accuracy as prognostic biomarkers in breast cancer (8,9). An examination of prognostic RNA profiles showed common profiles of cell cycle-regulated mRNAs (8,10). These medical utilities of a prognostic signature for breast cancer already have been widely used for patients (11). Recently, molecular subtype signatures have been developed to support for defining the risk of recurrence even in early stage lung cancer (12-16). However, as highlighted in a recent review article, many of these signatures are not independently verified, failed to show independence from clinical variables, or were not authorized on platforms suitable for patients in clinical (17). Furthermore, very few of these signatures have been fully evaluated in combination with clinicopathological variables, and even fewer have been handled in formalin-fixed clinical samples. To date, no gene signatures have been included in clinical practice guidelines such as NCCN guideline for the treatment of early stage lung cancer.
In order resolve this issue, several groups have developed gene expression analyses (Table 1) (18-21) with multiple strengths such as (I) a large NSCLC patient cohort with associated strong statistical power; (II) a homogenous patient population of stage I and II patients without adjuvant treatment; (III) a predesigned prognostic score and predesigned cut-offs for risk categories; (IV) specimens collected from several independent large centers in worldwide; and (V) reducing it into practice in a closer to the real clinical setting which is represented by real time quantitative polymerase chain reaction (RT-qPCR)-based platform suitable for the analysis of using routine clinical formalin-fixed paraffin-embedded (FFPE) tissue samples. Dama et al. (18) approved 10-gene (E2F1, E2F4, HOXB7, HSPG2, MCM6, NUDCD1, RRM2, SCGB3A1, SERPINB5, SF3B1) prognostic signature capable of identifying an aggressive molecular subtype of stage I lung ADC, with genetic characteristics very similar to advanced lung cancer. These 10-gene prognostic signature may be helpful to identify stage I patients who would benefit from multimodality adjuvant treatment. Before this validation study, their group previously described a 10-gene signature able to predict prognosis of 21 patients with stage I lung ADC (22). They included the three reference genes (TBP, HPRT1, and GUSB), used for the identification of the original 10-gene signature from fresh-frozen (FF) samples. The authors now developed and optimized a RT-qPCR-based six methods for assessing 10-gene signature using FFPE tissue samples. Using this protocol, the authors validated the prognostic accuracy of the 10-gene signature in an independent and large cohort of 507 lung ADC patients, including 351 stage I lung ADC patients. In this study, stage I patients (n=351) identified as high-risk by the 10-gene signature displayed a 4-fold increased risk of death (HR =3.98; 95% CI: 1.73–9.14), with a 3-year overall survival of 84.2% (95% CI: 78.7–89.7) compared to 95.6% (92.4–98.8) in low-risk patients. Furthermore, the authors performed additional integrated analysis of gene expression, methylation, somatic mutations, copy number variations, and proteomic profiles using a second independent cohort of 468 lung tumors profiled by The Cancer Genome Atlas (TCGA) (23). The analysis of TCGA cohort explained that the 10-gene signature identifies a subgroup of stage I lung ADCs demonstrating specific molecular characteristics and associated with aggressive performance and poor outcome. Incorporating these results, the 10-gene signature has been analyzed in total 1,487 lung ADC patients from three different large cohorts from Italy or from other countries, and across different platforms (507 by RT-qPCR on FFPE, 468 by RNA-sequence and 442 by Affymetrix, and 70 by RT-qPCR on FF specimen) (22).
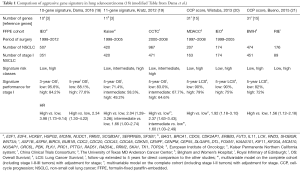
Full table
Recently, two other gene signatures were proposed for the stratification of early stage NSCLC (19-21). The characteristics of these signatures, in comparison with the 10-gene signature, are reported in Table. Kratz et al. (19) evaluated lung risk score, which uses quantitative PCR to examine the expression levels of 14 genes (BAG1, BRCA1, CDC6, CDK2AP1, ERBB3, FUT3, IL11, LCK, RND3, SH3BGR, WNT3A, ESD, TBP, YAP1) and three reference genes (ESD, TBP, YAP) in formalin-fixed tissue using two large cohorts of non-squamous operative NSCLC. The score was demonstrated to predict overall survival in non-squamous NSCLC, separating patients into groups with low risk, intermediate risk, and high risk of 5-year mortality in cohorts of mixed stages and in a sub set analysis of tumors <2 cm (24). The lung risk score study supports the idea that expression signatures can be established for use in formalin-fixed lung tissue and that they may have similar importance in modifying treatment decisions in lung ADC as breast cancer (8,9,25). This study distinguished itself from the 14-gene risk score by focusing on a gene set that is directly related to a well-established factor of tumor cells. However, neither the 14-gene signature nor any other expression profile has the achievement of sufficient acceptance to become the standard of care in clinical. Recently, the expression levels of cell-cycle progression (CCP) genes measure tumor growth regardless of the underlying genetic abnormality. CCP signature has been previously shown to be a superior prognostic tool in the treatment of prostate cancer (10). The expression levels of CCP genes illustrate that these gene profiles measure tumor growth irrespective of underlying histological grading, morphological grade, or genetic aberrations; this emphasizes the advantage of identifying a high risk cohort that may benefit from adjuvant chemotherapy that targets cell proliferation including a low risk cohort that can quit adjuvant therapies. CCP score has been previously shown to be a superior prognostic factor in early stage lung cancer as well as prostate cancer (20,21). Wistuba et al. previously reported that the development and validation of an mRNA expression signature of CCP genes to predict cancer related death from early stage lung cancer (20). A CCP score was measured from the mRNA expression levels of 31 proliferation genes (ASF1B, ASPM, BIRC5, BUB1B, CDC2, CDCA3, CDCA8, CDKN3, CENPF, CENPM, CEP55, DLGAP5, DTL, FOXM1, KIAA0101, KIF11, KIF20A, MCM10, NUSAP1, ORC6L, PBK, PLK1, PRC1, PTTG1, RAD51, RAD54L, RRM2, SKA1, TK1, TOP2A) in stage I and stage II lung cancer samples from two well-known public microarray datasets (Director’s Consortium and GSE31210) (20). The same gene set was tested by quantitative PCR in 381 FFPE primary tumors. In this analysis, the CCP score was a significant independent predictor of cancer related mortality in lung ADC in three independent large datasets. One limitation was that pathological stage remained an independent prognostic factor besides the CCP score. Then, they combined prognostic score of CCP and clinicopathological stage based on the data in the CCP validation study. The combined score combined molecular and clinical data to acquire a remarkable predictor of outcome than variable alone. Bueno et al. (21) further validate the association of CCP with 5-year lung cancer mortality after adjusting for clinicopathological factors. They also needed to investigate the prognostic score as a predictor of 5-year lung cancer mortality risk and to establish a cut off point for classifying patients into low risk and high risk groups. This study validates the CCP and prognostic score as powerful molecular biomarkers that predict cancer related death from early stage lung ADC and provide useful information to decide which patients need to be considered for additional multimodality therapy to improve survival. These approaches recognized the cooperative nature of molecular and clinical features.
To date, several molecular biomarkers have been identified that may predict tumor aggressiveness in patients with lung cancer. However, there is still argument about using gene signature to identify candidates for adjuvant treatment. For future clinical trials, it will be important to limit the number of favorable patients undergoing unnecessary treatment by carefully establishing the cut off used to classify patients at high risk. This decision should consider the adjustment between the numbers of over treated and under treated patients and may be relieved by the integration of multiple prognostic models.
In summary, this molecular biomarker assay provides a reproducible and quantitative measure of tumor aggressiveness that can provide important prognostic information to add on conventional clinicopathological prognostic factors. The use of these measurements may help management of stage I lung ADC by prompting investigations of benefits of adjuvant chemotherapy following curative surgical resection. We believe that molecular biomarkers will be helpful to provide useful prognostic information that may assist clinicians to decide whether to consider adjuvant therapy, schedule an ideal follow up a plan for patients and propose new clinical trials in the future.
Acknowledgments
Funding: Hideki Ujiie received a research scholarship from the Joseph M. West Family Memorial Fund.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Yi-Jiu Ren (Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.06.12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- National Lung Screening Trial Research T. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Ujiie H, Kadota K, Chaft JE, et al. Solid Predominant Histologic Subtype in Resected Stage I Lung Adenocarcinoma Is an Independent Predictor of Early, Extrathoracic, Multisite Recurrence and of Poor Postrecurrence Survival. J Clin Oncol 2015;33:2877-84. [Crossref] [PubMed]
- Pisters KM, Evans WK, Azzoli CG, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol 2007;25:5506-18. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043-51. [Crossref] [PubMed]
- Potti A, Mukherjee S, Petersen R, et al. A Genomic Strategy to Refine Prognosis in Early-Stage Non–Small-Cell Lung Cancer. New England Journal of Medicine 2006;355:570-80. [Crossref] [PubMed]
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351:2817-26. [Crossref] [PubMed]
- Eiermann W, Rezai M, Kummel S, et al. The 21-gene recurrence score assay impacts adjuvant therapy recommendations for ER-positive, node-negative and node-positive early breast cancer resulting in a risk-adapted change in chemotherapy use. Ann Oncol 2013;24:618-24. [Crossref] [PubMed]
- Cuzick J, Swanson GP, Fisher G, et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol 2011;12:245-55. [Crossref] [PubMed]
- Sparano JA, Paik S. Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol 2008;26:721-8. [Crossref] [PubMed]
- Shedden K, Taylor JMG, Enkemann SA, et al. Gene expression-based survival prediction in lung adenocarcinoma: a multi-site, blinded validation study. Nat Med 2008;14:822-7. [Crossref] [PubMed]
- Tang H, Xiao G, Behrens C, et al. A 12-Gene Set Predicts Survival Benefits from Adjuvant Chemotherapy in Non–Small Cell Lung Cancer Patients. Clinical Cancer Research 2013;19:1577-86. [Crossref] [PubMed]
- Kadara H, Behrens C, Yuan P, et al. A Five-Gene and Corresponding Protein Signature for Stage-I Lung Adenocarcinoma Prognosis. Clinical Cancer Research 2011;17:1490-501. [Crossref] [PubMed]
- Ujiie H, Lee D, Kato T, et al. Gene Signature. In: Takiguchi Y, editor. Molecular Targeted Therapy of Lung Cancer. Singapore: Springer Singapore, 2017:(279-92).
- Ujiie H, Kato T, Lee D, et al. Overexpression of MAGEA2 has a prognostic significance and is a potential therapeutic target for patients with lung cancer. Int J Oncol 2017;50:2154-70. [Crossref] [PubMed]
- Subramanian J, Simon R. Gene expression-based prognostic signatures in lung cancer: ready for clinical use? J Natl Cancer Inst 2010;102:464-74. [Crossref] [PubMed]
- Dama E, Melocchi V, Dezi F, et al. An aggressive subtype of stage I lung adenocarcinoma with molecular and prognostic characteristics typical of advanced lung cancers. Clin Cancer Res 2017;23:62-72. [Crossref] [PubMed]
- Kratz JR, He J, Van Den Eeden SK, et al. A practical molecular assay to predict survival in resected non-squamous, non-small-cell lung cancer: development and international validation studies. Lancet 2012;379:823-32. [Crossref] [PubMed]
- Wistuba II, Behrens C, Lombardi F, et al. Validation of a proliferation-based expression signature as prognostic marker in early stage lung adenocarcinoma. Clin Cancer Res 2013;19:6261-71. [Crossref] [PubMed]
- Bueno R, Hughes E, Wagner S, et al. Validation of a molecular and pathological model for five-year mortality risk in patients with early stage lung adenocarcinoma. J Thorac Oncol 2015;10:67-73. [Crossref] [PubMed]
- Bianchi F, Nuciforo P, Vecchi M, et al. Survival prediction of stage I lung adenocarcinomas by expression of 10 genes. J Clin Invest 2007;117:3436-44. [Crossref] [PubMed]
- . Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543-50. [Crossref] [PubMed]
- Kratz JR, Van Den Eeden SK, He J, et al. A prognostic assay to identify patients at high risk of mortality despite small, node-negative lung tumors. JAMA 2012;308:1629-31. [Crossref] [PubMed]
- Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 2010;28:1829-34. [Crossref] [PubMed]


