Human papillomavirus related anal squamous cell carcinoma survival: a systematic review and meta-analysis
Introduction
Anal squamous cell carcinoma (ASCC) is an uncommon malignancy of the anal canal and perianal skin area, with annual incidences ranging from 1.0 to 2.5 per 100,000 population in many western countries (1). Its incidence is increasing by approximately 2% yearly, particularly in females (2,3).
Although many risk factors for ASCC development have been identified, such as the inherited genetic predisposition, the molecular mechanisms related to the ASCC carcinogenesis remain under investigation (4). The effect of infectious agents in anal carcinogenesis has also been suggested as direct carcinogens or promoters. Infection with oncogenic human papillomavirus (HPV) types has been identified as a causal agent in a variety of human carcinomas, including those of the cervix, anogenital region and head and neck (5-7). As reported previously, the high-risk HPVs prevalence were 89.7% in cervical cancer (8), 29.5% in head and neck cancer (9), 22.2% in esophageal cancer (10) and 31.9% in colorectal cancer (11).
In a study of patients with cervical cancer receiving radiation therapy, HPV-positive patients have a significantly better survival (12). Some retrospective clinical studies have consistently proved that patients with HPV-positive head and neck squamous cell carcinoma (HNSCC) had a better prognosis than patients with HPV-negative tumors (13-16). Anus can be infected with these viruses in the same way as the oral cavity, tonsils, and pharynx; it is supposed that the histological similarities between the head and neck squamous epithelia and anus would suggest a similar association and clinical characteristics. The prognostic value of the HPV status has previously been investigated in patients with ASCC. However, the results are much controversial.
Therefore, this systematic review and meta-analysis is conducted to assess the effects of HPV infection on overall survival (OS) and disease-free survival (DFS)/disease-specific survival (DSS)/relapse-free survival (RFS)/progression-free survival (PFS) in ASCC patients.
Methods
Literature search strategy
A systematic search up to 30 Apr. 2017 was conducted in MEDLINE (via PubMed) and Excerpta Medica database (EMBASE) to identify relevant articles. Search terms included ‘‘human papillomavirus or HPV”, ‘‘anal cancer or anal neoplasms or anal carcinoma’’ combined with “prognosis or prognostic or survival or outcome”. Additional relevant references cited in retrieved articles were also evaluated. This meta-analysis was performed in accordance with PRISMA guidelines (see Table S1).
Inclusion and exclusion criteria
All papers were reviewed by two authors (Jian-Ning Yao and Hai-Ning Zhou) independently. Uncertainties and discrepancies were resolved by consensus after discussing with a senior researcher (Yan-Le Li). All studies included in the final meta-analysis satisfied the following criteria: (I) patients were pathologically diagnosed as ASCC; (II) OS or DFS/DSS/RFS/PFS as the outcome of interest; (III) reported HR estimates with their corresponding 95% CI (or sufficient data to calculate of these effect measure), and (IV) English articles. If the study was reported in duplication, the one published earlier or provided more detailed information was included. Review articles and editorials were included if they contained original data. Abstracts were excluded.
Quality assessment
The quality of each study was evaluated in accordance with the revised ELCWP scoring scale described by Steels (17). Each item was assessed using an ordinal scale (possible values: 2, 1, 0). The overall score evaluated several dimensions of the methodology, grouped into four main categories: (I) scientific design: 0–10; (II) laboratory methodology: 0–14; (III) generalizability: 0–12; (IV) results analysis: 0–8. The total scores ranged from 0 to 44. The final scores were expressed as percentages, ranging from 0% to 100%, higher values indicated a better methodological quality.
Data extraction
Two of the authors (Jian-Ning Yao and Hai-Ning Zhou) performed the data extraction from each article and discrepancies were resolved by consensus. For studies meeting our inclusion criteria, a standardized data extraction form was used to extract the following data: the first author’s name, year of publication, country of origin, study design, period of enrollment, the length of follow-up, characteristics of the studied population (sample size, age, stage of disease and treatment method), HPV detection methods, and HR estimates for OS or DFS/DSS/RFS/PFS with corresponding 95% CIs. When data for HR was not available, we extracted the total numbers of observed deaths and the numbers of patients in each group to calculate HR (18). Data were extracted by Engauge Digitizer version 4.1 (http://digitizer.sourceforge.net/) from the graphical survival plots when data were only available as Kaplan-Meier curves (19), then the estimation of the HR was performed by the described method (18).
Statistical analysis
The HR with 95% CI was used to compute the pooled HPV infections and the OS or DFS/DSS/RFS/PFS in ASCC patients. A fix-effect or random-effect model was used to pool the data, based on the Mantel-Haenszel method (20) and the DerSimonian and Laird method (21), respectively. These two models provide similar results when between-studies heterogeneity is absent; otherwise, random-effect model is more appropriate.
Cochrane Q test (P<0.10 indicated a high level of statistical heterogeneity) and I2 (values of 25%, 50% and 75% corresponding to low, moderate and high degrees of heterogeneity, respectively) was used to assess the heterogeneity between eligible studies, which test total variation across studies that was attributable to heterogeneity rather than to chance (22). Subgroup analyses for HPV infections and the OS or DFS/DSS/RFS/PFS in ASCC patients were subsequently carried out according to the study type, geographical region, number of patients, clinical stage, detection method, PCR primers, HPV type, treatment method, hazard ratio and ELCWP score. Sensitivity analysis was also conducted to assess the influence of each individual study on the strength and stability of the meta-analytic results. Each time, one study in the meta-analysis was excluded to show that study’s impact on the combined effect size. Funnel plot and Begg adjusted rank correlation test for funnel plot asymmetry were performed to test any existing publication bias.
All statistical analyses were performed using STATA version 12 for Windows (StataCorp LP, College Station, TX, USA). A two-tailed P<0.05 was considered statistically significant.
Results
Literature search
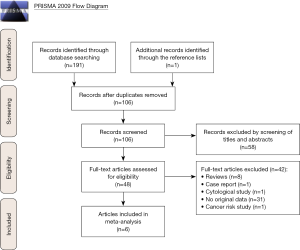
As shown in Figure 1, the search strategy generated 191 citations, of which 48 were considered of potential value after screening of titles and abstracts and the full text was retrieved for detailed evaluation. Of these 48 articles, 42 were subsequently excluded from the meta-analysis for various reasons, including 8 were reviews, 1 was case report, 1 was cytological study, 31 that did not provide HRs or CIs and 1 was cancer risk study. So, 6 studies were eligible and included in this systematic review and meta-analysis (23-28).
Characteristics of the selected studies
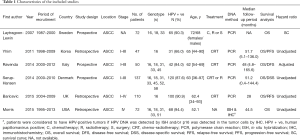
Individual characteristics of the included six studies are summarized in Table 1. They were published from 2007 to 2015 and involved a total of 488 ASCC cases. The sample sizes ranged from 47 to 137. All six studies investigated the prognostic role of HPV infection in OS, and 4 studies (23,26-28) explored the prognostic impact of HPV infection in DFS/DSS/RFS/PFS. HRs and 95% CIs were extracted directly from the survival curve from one study (24). One study (24) did not give accurate data for follow-up. The median follow-up period of all studies ranged from 28 to 51.7 months. The prevalence of HPV ranged from 66.0% to 94.4%.
Full table
Quality assessment
In methodological quality of studies, the global quality score ranged 56.8% to 77.3%, with a median of 69.0% (Table 2). The subscore of laboratory methodology had the lowest value, with a median quality score of 6.7 out of 14. The most poorly described items were the blinding evaluation, tissue sample conservation, and description of the revelation test procedure.

Full table
Results of the meta-analysis
OS
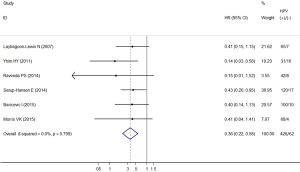
Among the studies included, all showed a negative association comparing HPV-positive to HPV-negative cancers, two (27,28) of which showed statistical significance. The heterogeneity test indicated there was very low degree of heterogeneity among included studies (Q test Pheterogeneity=0.799, I2=0.0%), thus a fixed effects model was employed to obtain the pooled HR. The pooled HR from the 6 individual effect estimates comparing HPV-positive to HPV-negative cancers was 0.36 (95% CI, 0.22–0.58), which was significantly correlated with improved OS (Figure 2).
DFS/DSS/RFS/PFS
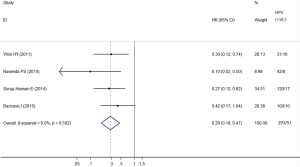
Among the studies included, all showed a negative association comparing HPV-positive to HPV-negative cancers, three (26-28) of which showed statistical significance. The heterogeneity test indicated there was low degree of heterogeneity among included studies (Q test Pheterogeneity=0.502, I2=0.0%), thus a fixed effects model was employed to obtain the pooled HR. The pooled HR from the four individual effect estimates comparing HPV-positive to HPV-negative cancers was 0.29 (95% CI, 0.18–0.47), which was significantly correlated with improved DFS/DSS/RFS/PFS (Figure 3).
Subgroup analyses
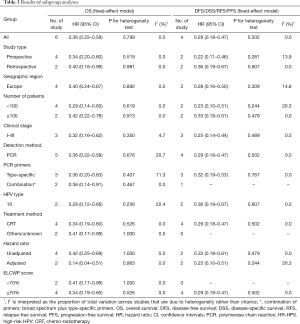
Table 3 presents detailed results of subgroup analyses.
Full table
The associations of HPV status and OS in ASCC patients did not differ by study type, geographical region, number of patients, clinical stage, detection method, PCR primers, HPV type, treatment method, hazard ratio and ELCWP score. When cancer cases stratified by HPV type, the pooled HR comparing HPV-16 positive to HPV-16 negative cancers was 0.28 (95% CI, 0.12–0.66).
The associations of HPV status and DFS/DSS/RFS/PFS in ASCC patients did not differ by study type, geographical region, number of patients, clinical stage, detection method, PCR primers, HPV type, treatment method, hazard ratio and ELCWP score. Exploratory subgroup analysis according to study type showed that HPV infection had more significantly prognostic value for improved DFS/DSS/RFS/PFS in prospective studies (HR =0.22, 95% CI, 0.11–0.46). In short, the estimated heterogeneity for studies included decreased to some degree but did not obliterate.
Influence analysis of individual studies
To address the potential bias due to the quality of the included studies, we performed the sensitivity analysis by calculating pooled HRs again when omitting one study at a time. Figure 4A,B showed the results of sensitivity analysis for OS and DFS/DSS/RFS/PFS respectively. The pooled HRs for OS comparing HPV-positive to HPV-negative cancers ranged from 0.32 (95% CI, 0.18–0.59) to 0.40 (95% CI, 0.24–0.66). The pooled HRs for DFS/DSS/RFS/PFS comparing HPV-positive to HPV-negative cancers ranged from 0.5 (95% CI, 0.14–0.44) to 0.32 (95% CI, 0.19–0.53). The meta-analysis result of the pooled HRs for OS and DFS/DSS/RFS/PFS comparing HPV-positive to HPV-negative cancers were not significantly affected by omission of any of the individual studies analysed, which indicated that each single study didn’t influence the stability of pooled HR estimate.

Publication bias
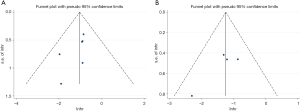
There was no evidence of publication bias as demonstrated by the non-significant P values of Begg’s test for OS (0.060) and DFS/DSS/RFS/PFS (0.142), and the near-symmetric funnel plot (Figure 5).
Discussion
This is the first systematic review investigating survival in HPV-related ASCCs. Pooled effect estimates from included studies demonstrated that HPV infection was significantly associated with improved survival in ASCC patients, suggesting that HPV infection may be of prognostic or therapeutic utility in the evaluation of factors contributing to ASCC.
The association between HPV infection and the occurrence of ASCC was first reported in the late 1980s (29-31). After then, an accumulating amount of studies have investigated the relationship between HPV infection and ASCC prognosis. However, the conclusions drawn were inconsistent. It was reported that HPV-positive HNSCCs was associated with a 54% reduction in overall mortality, in comparison to HPV-unrelated HNSCCs (32). Furthermore, Kim et al. (33) reported that the tumor HPV viral load in cervical cancer, in which more than 90% of patients had HPV infection, was a strong independent prognostic factor for DFS. In this sense, we are interested in the impact of tumor HPV status on treatment outcomes in terms of OS and DFS/DSS/RFS/PFS in ASCC. We found that HPV-positive ASCCs were associated with a 64% reduction in OS and a 71% reduction in DFS/DSS/RFS/PFS, in comparison to HPV-unrelated ASCCs. These findings could be explained with better responsiveness of HPV-positive ASCC to chemoradiotherapy, as already showed in oropharyngeal squamous cell carcinoma (26). However, it has not been well known why HPV positive tumors have better responsiveness to radiotherapy, chemotherapy or both and warrants further study.
There are more than 100 HPV genotypes, which are categorized low and high-risk in accordance with their ability to induce malignant transformation of epithelial cells (34). The overall prevalence of HPV in anal carcinoma is around 84.3%, wherein more than 75% and less than 10% were HPV 16 and 18 positive respectively (35). HPV-associated cancers often have a viral sequence integrated into the genome of the cancer cells. Two of the HPV early structural genes, E6 and E7, are known as oncogenes promoting tumor growth and malignant transformation. The E6 and E7 proteins contribute to the genetic instability through their inactivation of p53 and the retinoblastoma protein (pRb). pRb is a negative regulator of the cyclin-dependent kinase inhibitor p16, and inactivation of pRb leads to upregulation of p16. p16 is often used as a surrogate marker of HPV infection. Studies of HNSCC have demonstrated high concordance between expression of p16 and HPV positivity (13,36). In HNSCC, HPV and p16 status have been evaluated as prognostic factors with positive HPV status or increased p16 expression being associated with improved prognosis (37,38). One study of cervical cancer found that increased p16 expression was associated with a better prognosis (39). Few studies have evaluated HPV or p16 status as prognostic factors in patients with anal carcinoma (40). Results from subgroup analyses stratified by HPV type showed that HPV-16 infection had more significantly prognostic value for improved OS and less significantly prognostic value DFS/DSS/RFS/PFS. Future studies are encouraged to investigate the difference in survival between different HPV genotypes in ASCC.
Clinical stage at diagnosis is the most important prognostic factor for ASCC (41). It’s also a prerequisite for identifying ASCC patients who are candidates for chemoradiotherapy prior to surgery. However, only one study (26) reported the adjusted HRs for clinical stage. Other HRs were estimated either from univariate analysis or survival curves. So, future studies should therefore be encouraged to accurately adjust for other potential prognostic factors when comparing survival outcomes.
The present study has several strengths. First, the present analysis is the first to examine survival differences in HPV-positive and HPV-negative ASCCs, making it the most methodologically robust and comprehensive review to date. Second, we applied a rigorous inclusion/exclusion criterion, fully outcomes of interest (OS and DFS/DSS/RFS/PFS) and advanced meta-analysis of HR for survival. Moreover, subgroup analyses stratified by the study type, geographical region, number of patients, clinical stage, detection method, PCR primers, HPV type, treatment method, hazard ratio and ELCWP score. Thus, the effect of potential confounders was minimized. In addition, no publication bias was observed in our analyses, combined with the results of sensitivity analysis, indicating that our results are robust.
However, the present meta-analysis has several limitations. First, it is well known that the estimates of HPV infection might be influenced largely by the sensitivity and accuracy of HPV DNA detection method and HPV types covered by the method. Therefore, to some extent, potential bias could not be completely excluded considering that different methods have been used in the included studies. Second, the included studies were restricted to those published in English in our study, which might introduce language bias as well. Finally, only one study reported the adjusted HRs for clinical stage, which might cause residual confounding by other potential prognostic factors.
Conclusions
In conclusion, the findings of this meta-analysis indicated that HPV infection was significantly associated with improved survival in ASCC patients. Given its potential prognostic significance in ASCC, testing tumor specimens for HPV might indirectly affect the choice of chemotherapy and radiotherapy when considering treatment decisions. Considering the limitations of the present meta-analysis, further large prospective studies are encouraged to stratify survival analysis by pathological type and HPV type.

Full table
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.06.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van der Zee RP, Richel O, de Vries HJ, et al. The increasing incidence of anal cancer: can it be explained by trends in risk groups? Neth J Med 2013;71:401-11. [PubMed]
- Johnson LG, Madeleine MM, Newcomer LM, et al. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973-2000. Cancer 2004;101:281-8. [Crossref] [PubMed]
- Bilimoria KY, Bentrem DJ, Rock CE, et al. Outcomes and prognostic factors for squamous-cell carcinoma of the anal canal: analysis of patients from the National Cancer Data Base. Dis Colon Rectum 2009;52:624-31. [Crossref] [PubMed]
- Lynch HT, Lynch JF. Genetics of colonic cancer. Digestion 1998;59:481-92. [Crossref] [PubMed]
- Ciapponi A, Bardach A, Glujovsky D, et al. Type-specific HPV prevalence in cervical cancer and high-grade lesions in Latin America and the Caribbean: systematic review and meta-analysis. PLoS One 2011;6:e25493 [Crossref] [PubMed]
- Kreimer AR, Clifford GM, Boyle P, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 2005;14:467-75. [Crossref] [PubMed]
- Stelzer MK, Pitot HC, Liem A, et al. A mouse model for human anal cancer. Cancer Prev Res (Phila) 2010;3:1534-41. [Crossref] [PubMed]
- de Sanjosé S, Diaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis 2007;7:453-9. [Crossref] [PubMed]
- Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol 2014;15:1319-31. [Crossref] [PubMed]
- Zhang SK, Guo LW, Chen Q, et al. Prevalence of human papillomavirus 16 in esophageal cancer among the Chinese population: a systematic review and meta-analysis. Asian Pac J Cancer Prev 2014;15:10143-9. [Crossref] [PubMed]
- Damin DC, Ziegelmann PK, Damin AP. Human papillomavirus infection and colorectal cancer risk: a meta-analysis. Colorectal Dis 2013;15:e420-8. [Crossref] [PubMed]
- Harima Y, Sawada S, Nagata K, et al. Human papilloma virus (HPV) DNA associated with prognosis of cervical cancer after radiotherapy. Int J Radiat Oncol Biol Phys 2002;52:1345-51. [Crossref] [PubMed]
- Reimers N, Kasper HU, Weissenborn SJ, et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer 2007;120:1731-8. [Crossref] [PubMed]
- Syrjänen S. HPV infections and tonsillar carcinoma. J Clin Pathol 2004;57:449-55. [Crossref] [PubMed]
- Kong CS, Narasimhan B, Cao H, et al. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys 2009;74:553-61. [Crossref] [PubMed]
- Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J Clin Oncol 2006;24:736-47. [Crossref] [PubMed]
- Steels E, Paesmans M, Berghmans T, et al. Role of p53 as a prognostic factor for survival in lung cancer: a systematic review of the literature with a meta-analysis. Eur Respir J 2001;18:705-19. [Crossref] [PubMed]
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- MANTEL N. HAENSZEL W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719-48. [PubMed]
- DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemporary clinical trials 2007;28:105-14. [Crossref] [PubMed]
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21:1539-58. [Crossref] [PubMed]
- Baricevic I, He X, Chakrabarty B, et al. High-sensitivity human papilloma virus genotyping reveals near universal positivity in anal squamous cell carcinoma: different implications for vaccine prevention and prognosis. Eur J Cancer 2015;51:776-85. [Crossref] [PubMed]
- Laytragoon-Lewin N, Nilsson PJ, Castro J, et al. Human papillomavirus (HPV), DNA aberrations and cell cycle progression in anal squamous cell carcinoma patients. Anticancer Res 2007;27:4473-9. [PubMed]
- Morris VK, Rashid A, Rodriguez-Bigas M, et al. Clinicopathologic Features Associated With Human Papillomavirus/p16 in Patients With Metastatic Squamous Cell Carcinoma of the Anal Canal. Oncologist 2015;20:1247-52. [Crossref] [PubMed]
- Ravenda PS, Magni E, Botteri E, et al. Prognostic value of human papillomavirus in anal squamous cell carcinoma. Cancer Chemother Pharmacol 2014;74:1033-8. [Crossref] [PubMed]
- Serup-Hansen E, Linnemann D, Skovrider-Ruminski W, et al. Human papillomavirus genotyping and p16 expression as prognostic factors for patients with American Joint Committee on Cancer stages I to III carcinoma of the anal canal. J Clin Oncol 2014;32:1812-7. [Crossref] [PubMed]
- Yhim HY, Lee NR, Song EK, et al. The prognostic significance of tumor human papillomavirus status for patients with anal squamous cell carcinoma treated with combined chemoradiotherapy. Int J Cancer 2011;129:1752-60. [Crossref] [PubMed]
- Beckmann AM, Daling JR, Sherman KJ, et al. Human papillomavirus infection and anal cancer. International Journal of Cancer 1989;43:1042-9. [Crossref] [PubMed]
- Ostrow RS, Manias DA, Fong WJ, et al. A survey of human cancers for human papillomavirus DNA by filter hybridization. Cancer 1987;59:429-34. [Crossref] [PubMed]
- Palmer JG, Scholffield JH, Coates PJ, et al. Anal cancer and human papillomaviruses. Diseases of the Colon & Rectum 1989;32:1016-22. [Crossref] [PubMed]
- O'Rorke MA, Ellison MV, Murray LJ, et al. Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol 2012;48:1191-201. [Crossref] [PubMed]
- Kim JY, Park S, Nam BH, et al. Low initial human papilloma viral load implicates worse prognosis in patients with uterine cervical cancer treated with radiotherapy. J Clin Oncol 2009;27:5088-93. [Crossref] [PubMed]
- Muñoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003;348:518-27. [Crossref] [PubMed]
- De Vuyst H, Clifford GM, Nascimento MC, et al. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer 2009;124:1626-36. [Crossref] [PubMed]
- Lewis JS Jr, Thorstad WL, Chernock RD, et al. p16 positive oropharyngeal squamous cell carcinoma:an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol 2010;34:1088-96. [Crossref] [PubMed]
- Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24-35. [Crossref] [PubMed]
- Lassen P, Eriksen JG, Hamilton-Dutoit S, et al. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol 2009;27:1992-8. [Crossref] [PubMed]
- Schwarz JK, Lewis JS Jr, Pfeifer J, et al. Prognostic significance of p16 expression in advanced cervical cancer treated with definitive radiotherapy. Int J Radiat Oncol Biol Phys 2012;84:153-7. [Crossref] [PubMed]
- Ajani JA, Wang X, Izzo JG, et al. Molecular biomarkers correlate with disease-free survival in patients with anal canal carcinoma treated with chemoradiation. Dig Dis Sci 2010;55:1098-105. [Crossref] [PubMed]
- Kolligs FT. Diagnostics and Epidemiology of Colorectal Cancer. Visc Med 2016;32:158-64. [Crossref] [PubMed]