
Avelumab: a promising PD-L1 inhibitor for lung cancer (with inconvenient infusional reactions)
PD-1 and PD-L1 inhibitors have become an important treatment option for advanced non-small cell lung cancer (NSCLC) (1). In the first-line setting, a PD-1 inhibitor pembrolizumab by itself or in combination with chemotherapy has received a regulatory approval for marketing in the United States (2). In a previously treated setting, another PD-1 inhibitor nivolumab (3) and a PD-L1 inhibitor atezolizumab (4) have also been approved for clinical use. In addition, other agents which are already available in the United States for various indications include durvalumab (5) and avelumab (6). Durvalumab and avelumab are a PD-L1 inhibitor, currently approved for urothelial carcinoma; avelumab is also approved for Merkel cell carcinoma. Both drugs are being studied for clinical indications related to NSCLC. Although to date, there has been no head-to-head comparison between PD-1 and PD-L1 agents, it appears that PD-1 agent may be slightly more efficacious—as well as toxic—than PD-L1 agent based on the frequency of tumor responses and serious adverse events observed in NSCLC clinical trials. Nevertheless, it remains unclear how much this subtle difference will impact on overall survival and quality of life in the real world setting. At this time, these drugs are often considered as belonging to the same therapeutic class with potential cross resistance as well as cross efficacy. Within this increasingly crowded therapeutic arena, newer agents may be facing an identity crisis.
In a recent article, Gulley and colleagues reported on a phase-1 study of avelumab for recurrent metastatic NSCLC (7). This was a dose-expansion, phase 1b study. The dose-escalation, phase 1a study has been published separately (8). In the phase 1a study, the dosage 10 mg/kg given every 2 weeks was chosen as optimal and in this phase 1b study, 184 patients with previously treated advanced NSCLC were treated with the dosage and schedule. The results showed that there were 22 patients (12%, 95% CI: 8–18%) who achieved a confirmed tumor response: 1 complete response and 21 partial responses. The most common treatment related adverse events were fatigue in 25%, infusional reaction in 21% and nausea in 13%. The most common serious adverse event resulting in hospitalization was infusional reaction, reported in 4 out of 184 patients (2.2%). As expected, other immune-related adverse events from avelumab were infrequent and mostly mild, reported in 12% of patients, not much different from other comparable PD-1 or PD-L1 inhibitors used in this setting.
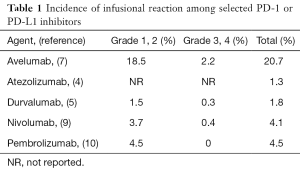
Nevertheless, what stands out for avelumab is the high incidence of infusional reaction. Infusional reaction has been uncommon in other available PD-1 or PD-L1 inhibitors. However, it occurred in about one fifth of patients treated with avelumab: Of 184 patients, 38 patients experienced an infusional reaction (20.7%). When putting this into the context of other agents from the same class, the incidence is about 14 times higher than other PD-L1 inhibitors and 5 times higher than other PD-1 inhibitors (Table 1). Grade 1 or 2 infusional reaction which was mild or severe enough to require interruption of the infusion but responding promptly to symptomatic treatment (e.g., antihistamines, non-steroidal anti-inflammatory agent, narcotics, intravenous fluids) occurred in 18.5% of patients, whereas grade 3 or 4, defined as prolonged (i.e., not rapidly responsive to symptomatic medication or brief interruption of infusion), recurrent after initial improvement or life-threatening occurred in 2.2%. Although there was no fatality, infusional reaction led to a permanent treatment discontinuation in 8 out of 184 (4%) patients. The onset of this complication was most frequently observed during the first or the second infusion, in about 90% of the times. The characteristics of reaction may include fever or shaking chills, flushing or itching, alteration in heart rate or blood pressure, dyspnea or chest discomfort, back or abdominal pain, nausea or vomiting or diarrhea, various types of skin rashes, typically resolving within 2 days. Despite the subsequent mandatory premedication with diphenhydramine and acetaminophen in this protocol, the reaction still occurred in 16% of patients and 2% of these events were grade 3 or 4.
Full table
What does infusional reaction really mean to the patient and staff? Although most oncology professionals are not unfamiliar with infusional reaction, each event still remains dramatic whenever it occurs. For the patient, the reaction immediately gives a negative first impression about the treatment, particularly when mind is set that this is not chemotherapy and there will be no acute ill effects such as nausea or vomiting. For the healthcare team, infusional reaction can be labor intensive and stressful to handle. Staff needs to interrupt the infusion and monitor closely whether the reaction will subside or take a turn for the worse. Severe cases may require paging of a code or rapid response team to ensure airway and circulation safety. Physicians will often be frantically reached for specific instruction or intervention. If the infusion is to be resumed, it needs to be restarted at a substantially slower rate, often extending the total infusion time by several hours. To other patients in the infusion center, it can at least cause anxiety, fear and at worst a disruption to their treatment schedule because of the incident. Since avelumab infusion is scheduled every 2 weeks and the reaction, once occurs, may keep recurring, the patient may need to relive the event every 2 weeks. Anyone can see how acceptable the treatment will be in the long run.
Beyond this inconvenient side effect, avelumab is a promising agent. Arguably the highest hope rests on its ability to induce an antibody-dependent cell-mediated cytotoxicity (ADCC) in vitro, thereby resulting in the avelumab-bound, PD-L1 expressing tumor cells being directly destroyed by immune cells (11). However, so far there has been no clear signal that avelumab works more rapidly or effectively than other agents in its class. It is also unclear if ADCC is necessary, given that the checkpoint is being blocked and tumor cells will be destroyed regardless. Numerous clinical trials on combination immunotherapy with avelumab are ongoing. Hopefully, avelumab may eventually find its niche in the therapeutic world for NSCLC.
History in oncology clearly shows that infusional reactions as serious as anaphylaxis will be put up with, when there is no other comparable treatment option. Although avelumab-associated infusional reaction may not be that serious, there are now many other comparable alternatives. In my opinion, the future of avelumab will heavily rely on an effective way to circumvent this troublesome reaction or an ability of avelumab to be remembered in some other positive ways than just another PD-L1 inhibitor with annoying infusional reactions, therefore best reserved only for Merkel cell carcinoma.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shaohua Cui (Department of Pulmonary Medicine, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.07.17). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tanvetyanon T, Gray JE, Antonia SJ. PD-1 checkpoint blockade alone or combined PD-1 and CTLA-4 blockade as immunotherapy for lung cancer? Expert Opin Biol Ther 2017;17:305-12. [Crossref] [PubMed]
- Pembrolizumab package insert. Available online: https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf
- Nivolumab package insert. Available online: https://packageinserts.bms.com/pi/pi_opdivo.pdf
- Atezolizumab package insert. Available online: https://www.gene.com/download/pdf/tecentriq_prescribing.pdf
- Durvalumab package insert. Available online: https://www.azpicentral.com/imfinzi/imfinzi.pdf#page=1
- Avelumab package insert. Available online: https://www.bavencio.com/en_US/document/Prescribing-Information.pdf
- Gulley JL, Rajan A, Spigel DR, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol 2017;18:599-610. [Crossref] [PubMed]
- Heery CR, O'Sullivan-Coyne G, Madan RA, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol 2017;18:587-98. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Boyerinas B, Jochems C, Fantini M, et al. Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti-PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells. Cancer Immunol Res 2015;3:1148-57. [Crossref] [PubMed]


