
Role of a selective cyclooxygenase-2 inhibitor on pain and enhanced recovery after open hepatectomy: a randomized controlled trial
Introduction
Postoperative pain has a significant impact on recovery and quality of life. Acute postoperative pain refers to intermediate pain lasting less than 7 days, but it will transform into chronic postsurgical pain with improper management. Several factors have been reported to influence of the development of postoperative pain, including age, sex, type of anesthesia, incision size, and type of surgery (1,2). Patients often have severe pain after open abdominal surgery with a large incision (3,4), and thus multi-modal analgesia including opioid and non-steroid anti-inflammatory drugs (NSAIDs) is commonly used following liver surgery. A single dose of opioids may not be ideal for gastrointestinal recovery because side effects such as nausea and vomiting are commonly seen after surgery. Selective cyclooxygenase (COX)-2 inhibitors have been shown to have analgesic efficacy in postoperative pain control and an opioid-sparing role in laparoscopic cholecystectomy and orthopedic surgery (5-7). However, there is little evidence on the efficacy of selective COX-2 inhibitors used in open abdominal surgery.
Liver hemangiomas are the most common benign tumor and usually asymptomatic. Giant hemangiomas are defined for diameter exceeds 4 cm, which meets the surgery indications (8). We analyzed postoperative pain control and recovery in the combination use of sufentanil and the highly selective COX-2 inhibitor parecoxib compared with traditional patient-controlled analgesia (PCA) using sufentanil. In our previous study, we discovered that patients in the parecoxib group showed significantly lower visual analogue scale (VAS) scores on the days 1 and 2 after surgery. Additionally, patients taking parecoxib administered less sufentanil on the postoperative day 3 and fewer patients in the parecoxib group required pethidine as a pain supplement for insufficient analgesia, which confirmed the efficacy for postsurgical pain management (Chen MT, Jin B, Du SD, et al., 2017, unpublished data).
Various studies on the mechanism of parecoxib for postoperative pain control have recently been conducted. Several studies showed higher plasma concentrations of pro-inflammatory factors including interleukin-6 (IL-6), IL-8, tumor necrosis factor (TNF)-α and hypersensitive C reaction protein (hsCRP) following acute or chronic pain, while plasma levels of anti-inflammatory factors such as IL-4, IL-10 and transforming growth factor (TGF)-beta decreased, which indicates a systemic inflammation response via release of different cytokines (9). Proinflammatory cytokines including IL-1beta, IL-6, IL-8, and TNF-α play an important role in mediating surgery-induced inflammation, especially 24 to 72 hours after major abdominal surgery (10). IL-10 inhibits the release of IL-1 and TNF-α from macrophages and monocytes. COX-2 and its downstream product, prostaglandin E2 (PGE2), are critical regulators for the inflammatory response. Additionally, recent research demonstrated that parecoxib inhibited IL-1beta and TNF-α expression via the COX-2/PGE2 pathway in the hippocampus of rats that underwent hepatectomy (11). The relationship between the cytokine cascade and inflammatory response remains controversial. Additionally, few studies focus on the surgery-induced inflammatory response after open hepatectomy. We hypothesized that the combination of parecoxib and opioids resulted in milder activation of surgery-induced systemic inflammation, which presented with a fluctuation in plasma levels of different kinds of cytokines. Thus, we further analyzed the plasma level of IL-1beta, IL-4, IL-6, IL-8, and TGF-beta to understand the relationship of cytokine release and postoperative pain control.
Methods
Patient selection and study design
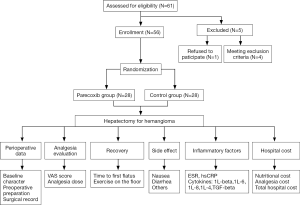
The study protocol was approved by the Institutional Review Board of Peking Union Medical College Hospital (PUMCH) and the approval number was S-685. All patients have signed the informed consent. The study outcomes did not affect the future management of the patients. The trial is registered at ClinicalTrials.gov (identifier NCT02204878). We enrolled hepatic hemangioma patients who were in the Department of Liver Surgery at PUMCH from September 1, 2014, to December 16, 2016. The study flow chart is presented in Figure 1. The inclusion criteria were adults aged 20 to 70 years old, without history of chronic pain, long term analgesia medication, drugs or alcohol abuse, and no reported history of allergy to NSAIDs, opioids, and sulfanilamide. Patients with active peptic ulcer, gastrointestinal bleeding, and inflammatory bowel disease were excluded.
Perioperative record
The method used for triple-blinded randomization is described below. Patients were enrolled randomly into the control group (intravenous PCA) or parecoxib group (intravenous PCA and parecoxib), and the randomization was generated from a randomization number obtained using SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA). Before anesthesia and after abdominal closure, each patient was given an unidentifiable, identical clear liquid prepared by the research pharmacist, which contained placebo [normal saline (NS) 2 mL] or the experimental drug (parecoxib 40 mg in 2 mL NS). For safety during surgery, pharmacists were unblinded to the specific regimen (NS or parecoxib) when preparing the medication. However, the nurse who was supervised by pharmacists was blinded to the regimen. Collectors were responsible for recording all information for eligible patients except the grouping results.
For the control group, PCA was established without background initial dosage using a lockout of 5 minutes and a 1-hour limit of 10 mL with 2 mL each time. For the experimental group, parecoxib 40 mg was administrated before anesthesia using the same set-up as in the control group, while parecoxib 40 mg every 12 h was given for 3 days after surgery. We recorded surgical details and detected blood chemistry profiles, and inflammatory markers [erythrocyte sedimentation rate (ESR) and hsCRP] before and after surgery. We recorded resting and active VAS, PCA dosage, pethidine for pain supplement, side effects (nausea and vomiting), time to first flatus, and exercise on floor after hepatectomy. We calculated each patient’s opioid use in sufentanil equivalents (mg), converting the dose of pethidine to opioid drugs depending on its analgesia effect. After the patient was discharged, the hospital cost was calculated. Details of the perioperative measurements were published previously (Chen MT, Jin B, Du SD, et al., 2017, unpublished data).
Blood sampling and processing
Blood samples were collected from a peripheral venous line at different time points, as follows: before surgery, and 48 and 72 hours after surgery. One blood sample was collected in 2-mL serum vials without anticoagulants and analyzed for levels of alanine aminotransferase (ALT), albumin (Alb), total bilirubin (TBIL), direct bilirubin (DBIL), creatinine (Cr), blood urine nitrogen (BUN), blood ammonia (NH3), ESR, and hsCRP. Another blood sample at each of the three time points was centrifuged at 3,000 revolutions per minute for 15 minutes. Serum was isolated after centrifugation and stored at −80 °C until ELISA assay for serum cytokines.
ELISA
Commercially available Quantikine enzyme-linked immunosorbent kits (R & D System, MN, USA) were used to determine serum cytokine concentrations (IL-1beta, IL-4, IL-6, IL-8, TGF-beta), according to the manufacturer’s instructions. ELISA results were obtained according to manufacturer’s procedures and using the ELISA reader (Synergy4 BioTek Inc., USA).
Statistical analysis
Results are presented as the mean ± standard derivation (SD). Student’s t-tests and Chi-squared tests were performed to compare continuous and discrete variables using the statistical software SPSS 19.0 (SPSS, Inc., Chicago, IL, USA). A value of P<0.05 was considered statistically significant and all tests was two-tailed.
Results
Clinical features
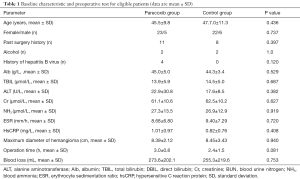
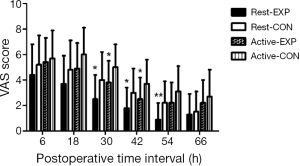
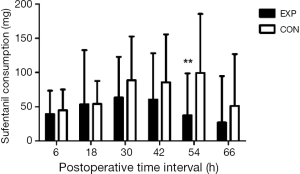
Sixty-one patients were assessed for study eligibility and 56 patients were ultimately enrolled (Figure 1). There were no statistically significant differences between the two groups in the perioperative demographic and clinical profiles, surgical time, and volume of blood loss (Table 1). For inflammatory markers, both ESR and hsCRP were increased in both parecoxib and control group after surgery. The ratio of postoperative and preoperative ESR at 42 hours after surgery was significantly reduced in the parecoxib group compared with control (P<0.05). We demonstrated that parecoxib provided greater relief than PCA following hepatectomy, with statistically lower VAS scores and sufentanil consumption as well as pethidine as pain supplement for insufficient analgesia in parecoxib group than control group (Figures 2,3). Fewer patients (17.8%) reported vomiting in the parecoxib group compared with the control group (Table 2). For further analysis, please refer to our previous study (Chen MT, Jin B, Du SD, et al., 2017, unpublished data).
Full table

Full table
Cytokine concentrations
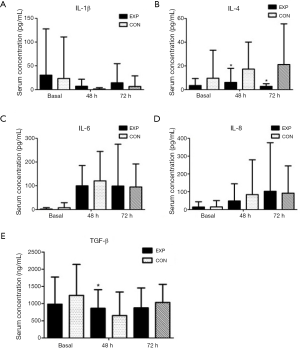
The concentration of the pro-inflammatory factor IL-8 remarkably increased after surgery in two groups (P=0.003). The pro-inflammatory factor IL-1beta and the anti-inflammatory factor TGF-beta significantly decreased at postoperative day 2 and 3 compared with baseline (P<0.0001 and P=0.001, respectively). The serum levels of the anti-inflammatory factor IL-4 and the inflammatory factor IL-6 were increased in both groups after surgery. The serum level of IL-1beta increased in the parecoxib group compared with the control group on days 2 and 3 after surgery, but the difference was also not significant (Figure 4A). Patients in the parecoxib group showed a significantly lower plasma concentration of the anti-inflammatory cytokine IL-4 at postsurgical days 2 and 3 (P=0.036 and P=0.011, respectively; Figure 4B). Compared with the control group, elevation of serum IL-6 decreased in the parecoxib group but the difference was not statistically significant (Figure 4C). At 48 hours after surgery, IL-8 levels decreased in the parecoxib group compared with the control group. However, 1 day later, the IL-8 concentration between the two groups became similar (Figure 4D). We also calculated the difference between pre- and postoperative plasma concentrations for all cytokines. The level of the anti-inflammatory factor TGF-beta was significantly increased in the parecoxib group after surgery (Figure 4E), indicating that there was more consumption of TGF-beta in the parecoxib group compared with the control group.
Discussion
Postoperative pain management following abdominal surgery is an important concern. Multi-modal analgesia including opioids, NSAIDs, and local nerve block around the wound can relieve discomfort and promote rapid recovery after surgery. Opioid drugs can effectively improve postoperative pain but have the disadvantage of a high incidence of nausea, vomiting, and constipation (12). Selective COX-2 inhibitors reduce opioid consumption as well as side effects of non-selective NSAIDs, especially platelet suppression and gastrointestinal reaction. Thus, selective COX-2 inhibitors have been used in mild to moderate analgesia or as one part of multimodal pre- and postsurgery analgesia. Recently, some randomized controlled studies confirmed the analgesia efficacy of selective COX-2 inhibitors on transcatheter arterial chemoembolization, maxillomandibular advancement surgery, and testicular surgery (5,13,14). Lin et al. enrolled 180 participants and compared VAS results and opioid consumption for 8 to 24 hours after laparoscopic cholecystectomy in parecoxib and placebo groups (7). He found that VAS, opioid consumption, and pain supplement were significantly reduced in the parecoxib group compared with the placebo group (7). Richebé drew a similar conclusion in pain management for total knee arthroplasty, where parecoxib reduced postoperative pain and adverse effects of opioids and also enhanced recovery after surgery (15).
However, few studies have focused on treating pain in patients undergoing open hepatectomy. Our study was the first prospective randomized controlled trial evaluating pain management in patients taking parecoxib for pain following open hepatectomy in China. In our previous research (Chen MT, Jin B, Du SD, et al., 2017, unpublished data), we showed the safety of parecoxib in patients with normal hepatic and renal function, and there was no hepatic or renal injury. The combination of parecoxib and opioids can effectively reduce postoperative pain by decreasing VAS scores and the number of patients required pethidine supplement. Parecoxib also reduced the vomiting incidence (46.4% and 64.3%, parecoxib group vs. control group), which was mainly caused by opioid drugs. In China, parecoxib is more expensive than opioids, but our study indicated that the total hospital fee did not significantly increase, while the fee for pethidine significantly increased in the control group.
Previous studies showed that postoperative pain was strongly associated with stimulation of the spinal dorsal root neuron by surgical trauma and release of inflammatory factors in local tissues (16). ESR and hsCRP were non-specific systemic markers of infection, tissue damage, and inflammation, and they were stimulated by several proinflammatory cytokines (9). To better understand the mechanism of parecoxib in pain management, we first investigated ESR and hsCRP to evaluate systemic inflammation and injury between the parecoxib and control groups. ESR and hsCRP significantly increased after surgery in both groups, which may indicate that open hepatectomy induced a moderate to severe systemic inflammatory reaction. We used the ratio of post-to-preoperative ESR to compare the extent of the increase in ESR and hsCRP in the two groups, and we found that the ESR was significantly lower in the EXP group compared with the CON group at 42 hours after surgery. This result suggests that parecoxib attenuated the inflammatory response to a certain degree. Studies in healthy adults revealed hsCRP was a sensitive biomarker for pain, and a higher level of hsCRP were often associated with higher pain sensitivity (17). On day 2 after surgery, VAS scores significantly decreased in the parecoxib group and the hsCRP level was 89.52±46.79 mg/L. Although this difference was 20.0 mg/L lower than in the CON group, it was not significant (P=0.173). This suggests that a selective COX-2 inhibitor was not sufficient for a severe inflammation response after open hepatectomy, which may be because open hepatectomy is an operation with one of the largest traumas. However, elimination of the inflammatory reaction required a few days, which was longer than was required to ameliorate postsurgical pain.
Recent studies demonstrated that pro-inflammatory cytokine levels remarkably increase in serum or interstitial fluid of patients with acute or chronic pain, while the level of pro-inflammatory cytokines decreased after analgesia therapy (15). IL-6 and IL-8, the main proinflammatory cytokines, are secreted by T helper 1 (Th1) cells in substantial quantities at the surgical wound site and they substantially enter the systemic circulation (10). Researchers found that parecoxib attenuated IL-6 and IL-8 production 24 hours after colorectal cancer surgery (18). Further research has demonstrated that an increase in the serum IL-6 concentration leads to stimulation of nociceptors and central sensitization, while an IL-6 inhibitor decreases hyperalgesia and allodynia (19). We suggest that IL-6 was associated with a surgery-induced inflammatory response. To further investigate the molecular mechanism of parecoxib on pain management, we analyzed the cytokine concentrations of IL-1beta, IL-4, IL-6, IL-8, and TGF-beta. Similarly, levels of pro-inflammatory cytokines including IL-6 and IL-8 remarkably increased after surgery while those of the anti-inflammatory cytokine TGF-beta decreased. In the parecoxib group, the concentration of serum IL-6 on day 2 after surgery was lower than in the control group, suggesting that parecoxib may reduce pain sensitivity by downregulating circulating IL-6. The postoperative level of anti-inflammatory IL-4 decreased in the parecoxib group and the difference between post- and preoperative TGF-beta concentration in the parecoxib group was higher compared with the control group. The results indicated that parecoxib might reduce the systemic inflammatory response through a mechanism other than only decreasing pro-inflammatory cytokine levels or increasing anti-inflammatory cytokine levels. The relationship between inflammatory cytokines and the COX-2 pathway remains ambiguous. In Wu’s study on cardiac surgery, he found that the increase of serum IL-6 and IL-8 concentration was attenuated by parecoxib 2 hours after surgery and a higher plasma concentration of the anti-inflammatory cytokine IL-10 was found one day after surgery (20). However, in another study, parecoxib balanced cytokines from several different T cells in patients who underwent laparoscopic cervical cancer surgery. Parecoxib suppressed the excessive production of IL-4 and IL-10 secreted by Th2 cells and reduced T regulator (Treg) cell production of TGF-beta. Parecoxib also increased IL-2 in Th1 cells 48 hours after surgery (21,22). In our study, IL-4 and TGF-beta levels were significantly lower in the parecoxib group compared with the control group at 48 and 72 hours after surgery, which might be explained by the immune-protective effect of parecoxib that balances cytokines from different T cells.
The advantage for our research is that it is the first prospective randomized controlled trial on postoperative pain management for open hepatectomy in patients receiving a combination of parecoxib and opioids in China. Additionally, we showed the efficacy and safety for parecoxib in hepatectomy. We also focused on the molecular mechanism of parecoxib to determine a specific biomarker of pain sensitivity, with which to evaluate parecoxib efficacy. However, our study had some limitations. We detected three time intervals that could be added to more closely track fluctuations, which can be used in further studies. Additionally, we only analyzed the serum cytokine concentration rather than the tissue concentration. However, it is difficult to detect the cytokine level in local injured tissue after surgery.
Conclusions
In summary, for open hepatectomy, combination use of parecoxib and opioids is safe and can effectively relieve pain and reduce adverse effect and hospital fees. For the molecular mechanism, parecoxib might attenuate the systemic inflammatory response to a certain extent, but the variation of cytokines requires further investigation.
Acknowledgments
Funding: This study was supported by Pfizer Pharmaceuticals Co., Ltd. (Shanghai, China). Grant numbers was WI187414.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Institutional Review Board of Peking Union Medical College Hospital (No. S-685). All patients have signed the informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Niraj G, Kelkar A, Kaushik V, et al. Audit of postoperative pain management after open thoracotomy and the incidence of chronic postthoracotomy pain in more than 500 patients at a tertiary center. J Clin Anesth 2017;36:174-7. [Crossref] [PubMed]
- Haroutiunian S, Nikolajsen L, Finnerup NB, et al. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain 2013;154:95-102. [Crossref] [PubMed]
- Ariche A, Klein Y, Cohen A, et al. Major hepatectomy for complex liver trauma. Hepatobiliary Surg Nutr 2015;4:299-302. [PubMed]
- Sapisochin G, Goldaracena N, Laurence JM, et al. Right lobe living-donor hepatectomy-the Toronto approach, tips and tricks. Hepatobiliary Surg Nutr 2016;5:118-26. [PubMed]
- Lv N, Kong Y, Mu L, et al. Effect of perioperative parecoxib sodium on postoperative pain control for transcatheter arterial chemoembolization for inoperable hepatocellular carcinoma: a prospective randomized trial. Eur Radiol 2016;26:3492-9. [Crossref] [PubMed]
- Schug SA, Joshi GP, Camu F, et al. Cardiovascular safety of the cyclooxygenase-2 selective inhibitors parecoxib and valdecoxib in the postoperative setting: an analysis of integrated data. Anesth Analg 2009;108:299-307. [Crossref] [PubMed]
- Lin S, Hua J, Xu B, et al. Comparison of bupivacaine and parecoxib for postoperative pain relief after laparoscopic cholecystectomy: a randomized controlled trial. Int J Clin Exp Med 2015;8:13824-9. [PubMed]
- de Werra E, Ettorre GM, Levi Sandri GB, et al. Major hepatectomy for a symptomatic giant liver cavernous hemangioma. Hepatobiliary Surg Nutr 2015;4:218-9. [PubMed]
- DeVon HA, Piano MR, Rosenfeld AG, et al. The association of pain with protein inflammatory biomarkers: a review of the literature. Nurs Res 2014;63:51-62. [Crossref] [PubMed]
- Lin E, Calvano SE, Lowry SF. Inflammatory cytokines and cell response in surgery. Surgery 2000;127:117-26. [Crossref] [PubMed]
- Peng M, Wang YL, Wang FF, et al. The cyclooxygenase-2 inhibitor parecoxib inhibits surgery-induced proinflammatory cytokine expression in the hippocampus in aged rats. J Surg Res 2012;178:e1-8. [Crossref] [PubMed]
- Arshad Z, Prakash R, Gautam S, et al. Comparison between Transdermal Buprenorphine and Transdermal Fentanyl for Postoperative Pain Relief after Major Abdominal Surgeries. J Clin Diagn Res 2015;9:UC01-4. [PubMed]
- Cillo JE Jr, Dattilo DJ. Pre-emptive analgesia with pregabalin and celecoxib decreases postsurgical pain following maxillomandibular advancement surgery: a randomized controlled clinical trial. J Oral Maxillofac Surg 2014;72:1909-14. [Crossref] [PubMed]
- Mehta A, Hsiao W, King P, et al. Perioperative celecoxib decreases opioid use in patients undergoing testicular surgery: a randomized, double-blind, placebo controlled trial. J Urol 2013;190:1834-8. [Crossref] [PubMed]
- Richebé P, Julien M, Brulotte V. Potential strategies for preventing chronic postoperative pain: a practical approach: Continuing Professional Development. Can J Anaesth 2015;62:1329-41. [Crossref] [PubMed]
- Kaminska B, Mota M, Pizzi M. Signal transduction and epigenetic mechanisms in the control of microglia activation during neuroinflammation. Biochim Biophys Acta 2016;1862:339-51.
- Schistad EI, Stubhaug A, Furberg AS, et al. C-reactive protein and cold-pressor tolerance in the general population: the Tromsø Study. Pain 2017;158:1280-8. [Crossref] [PubMed]
- Pandazi A, Kapota E, Matsota P, et al. Preincisional versus postincisional administration of parecoxib in colorectal surgery: effect on postoperative pain control and cytokine response. A randomized clinical trial. World J Surg 2010;34:2463-9. [Crossref] [PubMed]
- Zhou YQ, Liu Z, Liu ZH, et al. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation 2016;13:141. [Crossref] [PubMed]
- Wu Q, Purusram G, Wang H, et al. The efficacy of parecoxib on systemic inflammatory response associated with cardiopulmonary bypass during cardiac surgery. Br J Clin Pharmacol 2013;75:769-78. [Crossref] [PubMed]
- Ma W, Wang K, Du J, et al. Multi-dose parecoxib provides an immunoprotective effect by balancing T helper 1 (Th1), Th2, Th17 and regulatory T cytokines following laparoscopy in patients with cervical cancer. Mol Med Rep 2015;11:2999-3008. [Crossref] [PubMed]
- Sato N, Tamura T, Minagawa N, et al. Preoperative body mass index-to-prognostic nutritional index ratio predicts pancreatic fistula after pancreaticoduodenectomy. Hepatobiliary Surg Nutr 2016;5:256-62. [Crossref] [PubMed]


