
Convergence of nanotechnology with radiation therapy—insights and implications for clinical translation
Introduction
Ionizing radiation has been used for cancer treatments since the close of the nineteenth century, fairly soon after Wilhelm Roentgen discovered X-rays [1895], Henri Becquerel discovered radioactivity [1897], and Marie and Pierre Curie discovered radium [1898]. Since these early days of radiation therapy (RT), we have witnessed incremental changes and occasional quantum leaps in treatment techniques, paradigms, and machines.
Beginning with cathode-ray tubes and advancing through gantry-mounted cobalt heads in treatment machines, megavoltage linear accelerators, and charged particle accelerators, changes in technology have occurred in close parallel with similar advances in other technical disciplines like physics and engineering. Treatment delivery has been revolutionized by the use of motorized individually controllable collimator leaves that permit modulation of the intensity of radiation in real-time during treatment. Coupling the movement of these collimators to movement of the gantry now permits faster rotational arc treatment. Other forms of RT that have evolved over these years include the use of radioactive sources placed close to or within tumors (brachytherapy), electron radiation, and heavy ion radiation (largely protons and carbon ions).
As importantly, the clinical discipline has benefited from interaction with less technological but more clinical and biological disciplines. Growing up alongside diagnostic radiology, the field has co-opted much of the progress in imaging. Kilovoltage two-dimensional X-ray simulators have now been replaced with three- and four-dimensional computed tomographic (CT) simulators, magnetic resonance (MR) simulators, and positron emission tomographic (PET)-CT simulators to acquire images of tumors to aid the sculpting of beams aimed at them. These machines have also found their way into the treatment room and are an integral component of image-guided delivery systems. The other discipline that developed alongside RT was tumor biology (radiobiology), bringing with it the concept of optimizing the therapeutic ratio where the intent of treatment was maximal tumor control with minimal collateral damage to adjacent normal tissues. More importantly, the modern practice of RT was founded on the recognition that greater tumor control with less normal tissue toxicity can be achieved by fractionating treatments into smaller instalments rather than delivering all of it as a single large dose. In a departure from this conventional wisdom, assimilation and incorporation of sophisticated image-guided delivery techniques have resulted in increasing acceptance of short-course high-dose (stereotactic) radiation treatments. This is again a testament to the convergence of a greater understanding of radiation biology and the emergence of newer enabling technologies. These conceptual advances have benefited immensely from encounters with mathematical and statistical modelling techniques that allow prediction of the behavior of radiated tissues a priori. The other discipline that has grown alongside RT in the latter half of the last century is medical oncology. Increasingly, RT is interwoven with chemotherapy (concurrently and/or sequentially) to increase therapeutic efficacy without excessive toxicity. The latest entrant in this crosstalk between disciplines is the explosion in our knowledge of the biological hallmarks of cancer at the genetic and molecular level. Molecular biology continues to refine the way RT is chosen for subsets of patients with specific molecular traits, tailored to the intrinsic make-up of an individual patient’s tumor, often adapting to inducible changes, or combined with molecularly targeted agents for maximum therapeutic benefit.
As illustrated above, the history of radiation oncology is replete with examples of solving research problems with multidisciplinary approaches that bridge disparate life science and physical science fields. It is within this context that we view radiation oncology’s convergence with nanotechnology the study and manipulation of matter and phenomena at a nanoscale, about 1 to 100 nm. Most applications of nanotechnology in medicine (as elsewhere) harness the unique physical and chemical properties of matter at these size regimes compared to bulk matter, and employ these particles to sense, image, measure, and manipulate biologic processes and functions. These characteristics largely arise from the large surface area to volume ratio and the tunable intense and narrow spectral absorption and scattering cross-sections when interacting with electromagnetic waves. In turn, the large surface area to volume ratio translates to greater potential for interaction with biomaterials and surfaces than single molecules, greater potential for decoration of their surface with targeting, imaging and/or therapeutic agents, and greater ability to multiplex different functionalities (an exciting new field of theranostics, i.e., the merger of therapeutics and diagnostics, borrows heavily from these properties of nanoparticles). The strong absorption and scattering properties can be clinically exploited to amplify a weaker signal from individual molecules (for instance, Raman scattering), or generate heat (for instance, plasmon resonance) that could itself be used for therapeutic purposes or could be imaged by photoacoustic imaging. From the perspective of cancer imaging and therapy, a unique feature of the tumor itself that makes it accessible to nanoparticles is the presence of leaky, immature and chaotic blood vessels with fenestrations ranging from 60-400 nm when compared to the surrounding healthy tissues. The consequent tumor-specific accumulation of intravenously administered nanoparticles is called the enhanced permeability and retention (EPR) effect, wherein nanoparticle leak out through these fenestrations and are retained within the disorganized extracellular architecture of tumors.
Nanoparticles can be fabricated with different sizes, shapes, and surface properties from numerous materials. Although organic molecules like polymers and liposomes have also found broad applicability in radiation oncology and are further along in clinical trials, this review highlights the potential for and the challenges to realizing similar clinical advances with metallic nanoparticles as conduits to improving RT.
Nanoparticle-mediated radiosensitization
Despite being an effective component of modern cancer therapy for localized disease, the ultimate utility of RT is limited by the fact that some cancer cells are resistant to ionizing radiation. Attempts to improve outcomes of RT have largely focused on (I) increasing the dose of radiation delivered to the tumor; (II) sensitizing the radioresistant fraction of tumor cells to conventional doses of radiation; and (III) targeting cancer cells specifically while administering RT. The advent of nanotechnology in the field of biology and medicine presents versatile opportunities to overcome the limitations associated with these traditional strategies by combining multiple approaches in one unified seamless therapeutic strategy. We address some of these strategies below.
Thermoradiotherapy with metallic nanoparticles
One of the key mediators of inherent radiation resistance of tumor cells is intra-tumoral hypoxia that contributes to changes at the genetic, epigenetic and protein levels within tumor cells and tumor micro-environmental changes that eventually result in greater tumor aggressiveness. Mild temperature (<43 °C) hyperthermia is a well-recognized therapeutic adjunct to conventional RT (thermoradiotherapy) (1-3) that exerts its radiosensitizing effects, in part, via enhanced vascular perfusion of tumors and consequent better oxygenation and reduced hypoxia (4). Despite its proven biologic and clinical efficacy, this strategy has not been widely adopted in the clinic because conventional methods of generating hyperthermia have been at least minimally invasive, lacking in means to monitor temperature non-invasively, and difficult to control and administer in a consistent and controlled manner. Current approaches to delivery of heat to tumors are based on methods which focus energy from outside the body to the tumor, like hot water bags, ultrasound, microwave, etc(5). These approaches result in uneven temperatures within the tumor with “heat-sinks” along vasculature where cooler blood dissipates heat efficiently from the heated adjacent tumor parenchyma. Metal nanoparticles, particularly gold and iron, offer an alternative approach to tumor heating.
Gold nanoparticles (GNPs) offer a radically different approach to induce mild temperature hyperthermia in tumor tissues. GNPs have a ‘cloud’ of free electrons whose oscillatory motion is restricted by the shape and size of the particle, giving rise to quantized waves called polaritons. When light (electromagnetic energy) of a specific wavelength is incident on gold nanoparticles such that the incident light photons are resonant with the polaritons, the electrons absorb the incident energy to become highly energized (plasmons). This energy is then released to the immediate environment in the form of heat. This phenomenon (surface plasmon resonance) results in a net transduction of light energy to heat energy (6). Since the resonant wavelength depends on the shape and size of the nanoparticle, and since light in the near infrared (NIR) region of the electromagnetic spectrum has the greatest penetration depth in human tissues, two types of GNPs—gold nanoshells and gold nanorods with plasmon resonance tuned to peak in the NIR region have been extensively used in pre-clinical investigations, in anticipation of eventual clinical translation (7). In the case of the silica-gold core-shell nanoparticles (gold nanoshells), the ratio of the thickness of the gold shell to the diameter of the dielectric silica core can be varied to tune the plasmon resonance to the NIR region. In the case of the solid cylindrical gold nanorods, the ratio of the length to the diameter can be varied to tune the longitudinal plasmon resonance to the NIR wavelength. Seminal report on the use of gold nanoshell-mediated thermoradiotherapy demonstrated integrated antihypoxic and localized vascular disrupting effect resulting in an enhanced RT response in mouse tumor model (8). The vascular disruption effect is mediated by the sequestration of gold nanoshells (NIR activatable ones are roughly 150 nm in size, comprised of a 120 nm diameter silica core and a 15 nm thick gold shell) in the perivascular zone where temperature rise adjacent to the nanoshells is considerably more than that within tumor parenchyma which reaches mild hyperthermia range temperatures. This heterogeneity of temperature within tumors is distinctly different from that encountered when hyperthermia is generated from the “outside in” and results in “cold spots” or “heat sinks” along blood vessels. Here, the temperature increase is generated at the blood vessel-tumor interface and dissipates from the “inside out”, thereby creating a “hot spot” along blood vessels. This not only results in vascular disruption but also ensures that the maximal heat is generated inside the targeted tumor with minimal heating of normal tissues and potentially accounts for the preferential sensitization of cancer stem cells residing in the perivascular niche to radiation (9). Nevertheless, the limited penetration depth of NIR light in tissues remains a great challenge reiterating the need for appropriate clinical scenarios to utilize this strategy to its maximum advantage. Superficial tumors (head and neck, skin, cavity locations reachable by endoscopes), low-attenuation tissues (e.g., breast) or post-surgical tumor beds (e.g., post-mastectomy) present ideal clinical scenarios for gold nanoparticle mediated thermoradiotherapy.
Alternatively, ferromagnetic nanoparticles that are made of various formulations of iron and/or iron oxide are ideal candidates to induce mild-temperature hyperthermia in deep-seated tumors where an external alternative magnetic field is used to activate these particles (10,11). The alternating field heats up the ferromagnetic nanoparticles through a combination of rapid hysteresis and Neel relaxation (12,13). An additional advantage is that these particles can be imaged using magnetic resonance imaging (MRI). Although this strategy seems to be promising to heat up deep-seated tumors the major challenge associated with this method is the requirement of large amount of ferromagnetic nanoparticles to generate sufficient heat for clinical applications. Water-soluble 15 nm particles with magnetic cores and silane coats that are directly injected into tumors (and visualized by MRI for thermal dosimetry purposes) have currently obtained approval in Europe for multiple clinical trials with at least one (glioblastoma model) having completed Phase II evaluation (14-16). These approaches to thermoradiotherapy have evolved in parallel with the more widespread availability of MR thermal imaging for non-invasive monitoring of temperature and the emergence of closed-loop hyperthermia generating and thermal imaging systems (such as the MR guided focused ultrasound systems) for controlled and consistent hyperthermic treatments.
Gold nanoparticle mediated radiation dose enhancement
The biological effect of radiation interaction with tissues is generally related to the linear energy transfer (LET)—defined as the amount of energy transferred per unit distance travelled in tissues which in turn depends on the kinetic energy of electrons (17). Since the normal and tumor tissues have similar electron densities, precise treatment planning is required (as widely adopted in current RT treatment strategies), to deliver maximum dose to the tumor tissues with minimum collateral damage to normal tissues. As a corollary, enhancing the electron density in the tumor tissues could potentially have favorable benefits in improving RT treatment outcomes. Electron dense high atomic number (Z) elements offer an excellent choice to enhance the radiation interaction cross-section of the target tissues (18). Combining this characteristic of high-Z elements with the unique tumor specific accumulation of nanoparticles (in the range of 1-100 nm) opens up the prospect of delivering greater radiation dose to tumors while sparing adjacent normal tissues. Although several high-Z elements have been explored for radiosensitization, GNPs have favorable characteristics such as biocompatibility, ease of conjugation, evasion of the immune system upon PEGylation and preferential accumulation in tumors by the EPR effect (19-22). Furthermore, active targeting via decoration of these GNPs with tumor-homing moieties (peptide, antibodies, oligonucleotides, etc.) affords tumor-specificity and the potential for internalization of GNPs into tumor cell cytoplasms and possibly nuclei. In turn, the presence of GNPs in tumor cells leads to (I) physical dose enhancement induced by the interaction of secondary electrons, generated from the GNPs (often localized in the peri-nuclear region of the cell), with the nuclear DNA and (II) enhanced biological response induced by the short-lived reactive oxygen species (ROS) generated near critical organelles within the tumor cell.
Physics of gold-mediated radiation dose enhancement
When atoms are irradiated with photon energies above the ionization energy of the innermost (K) shell electrons, photoelectric absorption results in the production of photo- and Auger/Coster-Kronig electrons (23,24). Classical photoelectric interactions occur when a high-energy photon collides with an atom to eject an electron—the photoelectron—from its shell; the remaining energy (incident photon energy minus energy transferred to the photoelectron) brings the whole atom to an excited state. This excess energy is released through two mechanisms that eject photons or electrons: X-ray fluorescence and ejection of Auger electrons. In both cases, the ejected particles form tracks of ionization in the tissue. In the case of high-Z nanoparticles, these secondary particles locally enhance the physical dose delivered around the metallic nanoparticles (25). Typically, photoelectric phenomena are dominant at kilovoltage (kV) energies and directly proportional to Z3-4 of the material. Consequently, the photoelectric cross-sections of high-Z materials (like gold, with a Z of 79) are considerably more than that of materials such as soft tissues containing carbon (Z=6), hydrogen (Z=1), nitrogen (Z=7) and oxygen (Z=8). Computational studies have shown that the yield of electrons is increased up to 10 times when 0.1% w/w of GNPs are incorporated into biological tissue irradiated with kilovoltage radiation beams (energy <200 keV), and approximately double when the same tissue is irradiated with megavoltage clinical beams—photons with energy up to 6 MeV (26). Studies have also shown the feasibility of using Yb-169 brachytherapy sources (matching the gold K-edge energy absorption) in combination with GNPs that demonstrated a dose enhancement of 2 orders of magnitude (27). While several macro, micro and nano-scale computations have demonstrated the radiation dose enhancement of gold using multiple photon sources (125I, 103Pd, 169Yb, 192Ir, 50 kVp, 6MV X-rays) there is no clear consensus on the optimal parameters to define the effectiveness of GNP-mediated radiosensitization (27-29). More recently, it has been demonstrated that the effectiveness of GNP-mediated radiosensitization depends on the size of GNPs, the rate of photoelectric absorption, the characteristics of the escaping Auger electrons and the location of GNPs within the cell (30,31). Despite these extensive computational investigations a complete understanding of the nanoscale effects of GNP-mediated radiosensitization remains a lingering question. Nevertheless, experimental evidence demonstrates excellent radiosensitization effects that are attributed to the biological consequences of the GNP-mediated physical radiation dose enhancement.
Biological mechanisms of gold nanoparticle radiosensitization
The interaction of ionizing radiation with tissues causes damage by depositing energy directly to biomolecules (direct effects) or by the producing ROS through radiolysis of water (indirect effect) via, superoxide (O.2-), hydrogen peroxide (H2O2) and hydroxyl (.OH) radical. In turn, these ROS generate DNA strand breaks, the most challenging ones to repair being double strand breaks (DSBs). A fine balance between DNA damage (primarily DSBs) and DNA repair is generally considered the primary determinant of the intrinsic radiosensitivity of tissues. Consequently, the effectiveness of GNP-mediated radiosensitization is evaluated by correlating the experimental outcome with the number of unrepaired DNA DSBs. A direct correlation between the cellular damage and the number of radiation-induced γH2AX and 53BP1 foci (markers of unrepaired DSBs) was reported for 50 and 2 nm GNPs; the damage induced by 50 nm GNPs is dependent on the cellular internalization of GNPs (32,33). A more recent investigation revealed 1.7 fold enhancement in γH2AX-foci at 24 hr after irradiation of glioblastoma cells incubated with 12 nm GNPs (34). While DNA DSBs are considered as the primary markers for radiation-induced cellular damage, some studies have demonstrated the role of intracellular ROS and apoptosis in GNP-mediated radiosensitization. Elevated levels of intracellular ROS and apoptosis have been reported in ovarian cells and breast cancer cells that were treated with 14 and 1.9 nm GNPs followed by kV and MV X-ray radiation (32,35,36). Additionally, the activation of cell cycle checkpoints in G1/S and G2/M phases, which maintain genomic integrity by repairing defects or preventing cell division, is a common response to ionizing radiation. Cells incubated with 10.8 nm glucose capped GNPs and irradiated with a 137Cs source demonstrated accelerated G0/G1 transition and subsequent accumulation in G2/M phase (37). Similar findings were observed in ovarian cancer cells irradiated with 6 MV X-rays following the incubation with 14 nm glucose capped GNPs (35). These experimental results suggests that factors such as modulation in cell cycle kinetics, the ability of the cells to recover from DNA/mitochondrial damage or from high levels of oxidative stresses in the cytoplasm contribute to the effectiveness of GNP-mediated radiosensitization. Even without accounting for the heterogeneity of cell populations (stem cells, endothelial cells, immune and hematopoietic cells, hypoxic cells, etc.) in vivo within tumors and their differing responses to radiation, these cellular effects go beyond the predictions of physical dose enhancement to modify the biological effect of a given form of radiation on tumors laden with GNPs.
Pre-clinical evidence and outlook for clinical implementation
A seminal report on GNP-mediated radiosensitization in animal tumor models demonstrated a remarkable 1-year survival rate of 86% following followed by 26 Gy radiation with 250 kVp X-ray when mouse tumors were laden with 1.9 nm intravenously administered GNPs vs. 20% for tumors not laden with GNPs. Based on this promising result, subsequent in vitro and in vivo studies were conducted to investigate both the enhanced intracellular damage and the global tumor response to RT in the presence of GNPs. Attempts to demonstrate the feasibility of using clinically relevant radiation beams showed delayed tumor growth and increased apoptosis in mice injected intravenously with 13 nm GNPs, 24 hr prior to a radiation dose of 25 Gy from a 6 MV clinical accelerator. When combined with hyperthermia, the therapeutic outcome of GNP-mediated radiosensitization was enhanced in radiation resistant squamous cell carcinomas (38). More recent investigations on the combination of GNPs and proton radiation (40 MeV, 10 to 41 Gy) demonstrated 1-year survival of 58-100% with GNPs and 11-13% without GNPs in murine CT26 colorectal cancer models (39,40). Thus, convincing pre-clinical evidence along with in vitro studies suggests that radiation dose enhancement by GNPs can be accomplished using multiple types of radiation (photons, protons, electrons) from different sources (kilovoltage and megavoltage X-rays, HDR brachytherapy, protons) with different energies (low energy kilovoltage ranging from 50-300 kVp and high energy megavoltage ranging from 6 to 160 MV) (41). Although gold nanoparticle mediated radiation therapy (GNRT) is predominantly dependent on the energy of the radiation, with clinically less significant low energy beams being more efficient in generating secondary electrons when compared to the high energy beams, the therapeutic outcome of the clinically relevant high energy megavoltage beams can be modulated by enhancing tumor-specific localization of the GNPs.
The vast majority of pre-clinical investigations accomplish tumor-specific localization of GNPs via passive targeting that is dependent on the GNP size and the EPR effect. Larger GNPs tend to extravasate and accumulate in the perivascular space without penetrating deep into tumor parenchyma or getting internalized within cells. In contrast, smaller GNPs with enhanced permeability and diffusion characteristics demonstrate enhanced accumulation within tumor tissues (1% w/w) and may be internalized by some tumor cells. These present an ideal choice to transiently increase the radiation interaction cross-section of tumors. However, very small GNPs often act as intravascular contrast agents and are rapidly extruded from vasculature into tumors and equally rapidly efflux back into circulation due to the high interstitial tumor pressure within tumors. This rapid tumor uptake and immediate wash-out necessitates delivery of radiation immediately after the intravenous infusion of GNPs for effective radiation dose enhancement. The short interval (~2 min) between GNP administration and the radiation dose delivery, and the need for such administration before each radiation fraction reduce the enthusiasm for this approach in the clinic. Therefore, for clinically meaningful radiation dose enhancement, an approach that achieves the sustained presence of GNPs at high concentrations within the tumors is desirable. This could be accomplished by the active targeting strategy where the GNPs can be conjugated to antibodies or peptides directed against tumor antigens or antigens on tumor vasculature for tumor-specific localization of these GNPs. Thereafter, receptor-mediated or other non-specific methods (caveolin-mediated, macropinocytosis, etc.) may cause internalization that could bring these GNPs within close proximity to DNA, mitochondria, and cell membranes where short-range secondary electrons emanating from irradiated GNPs could cause DNA DSBs, mitochondrial membrane depolarization or lipid peroxidation, respectively. Additionally, the intracellular localization of GNPs achieved via active targeting could potentially minimize the amount of GNPs required to induce substantial radiation dose enhancement during GNRT, with less collateral damage to surrounding normal tissues. A more recent investigation using Her2-conjugated GNPs (~54 nm) and 100 kVP X-rays demonstrated a tumor regression of breast cancer models by 46% as compared to treatment with radiation alone (42). Our unpublished data with gold nanorods conjugated with an anti-EGFR antibody or a luteinizing hormone releasing hormone (LHRH) agonist strongly support this hypothesis. Nevertheless, uncertainties related to the intratumoral biodistribution of GNPs due to the heterogeneity of EPR in tumors still persist. While attempts to delineate the parameters for an enhanced biodsitrbution within tumors have largely been confined to pre-clinical investigations using animal tumor models, understanding the EPR effect in humans remains elusive. In particular, various parameters such as the variability in tumor vascular architecture, pore dimensions within and between tumor types, the location and origin of the tumor, nature of the vascular bed and surrounding stroma, tumor size, etc. could significantly influence the heterogeneity of EPR in tumors. Hence, integration of GNP imaging with GNP treatment would not only monitor and quantify the GNP biodistribution within tumors but also permit dosimetry and prediction of effects with biophysical models that include physical dose enhancement principles and biological parameters that modify these effects.
Nanoparticle-mediated delivery of radiosensitizing drugs to tumor
Extensive studies have been devoted to address the radiation resistance of tumors using multiple potent chemical radiosensitizers (43). The well-known radiosensitizers which work through chemical or biochemical means are hydroxyurea, halopyrimidines 5-iododeoxyuridine (IUDR) (44), 5-bromo-2'-deoxyuridine (BUDR) (45), 5-fluoro-2'-deoxy-beta-uridine (FUDR), trans sodium crocetinate, hypoxic radiosensitizers (nitric oxide, tirapazamine, nitroimidazoles like nimorazole, and the anthraquinone AQ4N), topoisomerase inhibitors (camptothecin and topotecan), alkylating agents (temozolomide) and monoclonal antibodies (cetuximab). Other examples of common radiosensitizers (also used as chemotherapeutic agents) are 5-fluorouracil (5-FU), doxorubicin, taxanes, gemcitabine, and platinum-based drugs (cisplatin and carboplatin).
One major difficulty in the implementation of these agents in radiotherapy as radiosensitizers is their cytotoxic effects and off-target effects in normal tissues (46). Such limitations can potentially be overcome by designing carriers with multi-faceted characteristics that include encapsulation and controlled release, minimization of immune-clearance, penetration of biological barriers and targeting the disease site (47,48). Various micro- and nano-sized carrier systems (liposomes, nanoparticle albumin-bound (Nab), polymeric nanoparticles, dendrimers, inorganic metal nanoparticles, and molecular targeted nanoparticles) (49) have been designed to create extended release formulations of drugs at the target site, while decreasing overall systemic drug dose to levels below the toxicity threshold (50,51).
Radioimmunotherapy using nanoparticles
Radionuclides formulated as nanoparticles have the potential to accumulate preferentially within tumors through passive diffusion or active targeting and thereby irradiate from within. Liposomal formulation of radionuclides (52) contributes to passive tumor accumulation via the EPR effect. For preventing hepatic accumulation, pretreatment with non-radioactive liposomes was effective in an in vivo biodistribution study (53). Utilization of tumor-specific metabolic and immune processes provides an effective route to deliver radionuclides preferentially to tumor tissues. Radioactive iodine has been used in the treatment of thyroid cancer due to its characteristic of being mostly taken up by the thyroid gland after systemic administration. In radioimmunotherapy, radionuclides are attached to antibodies to target tumors. Instead of attaching one radionuclide to each antibody, producing nanoparticles containing hundreds of radioactive atoms was demonstrated to deliver up to 50 Gy to tumor cells with Y2O3 nanoparticles (54). Yttrium-encapsulated 8 nm apoferritin shells with biotin surface modification was shown to be an effective strategy to conjugate multiple antibodies, constructing radioactive nanoparticles for radioimmunotherapy applications (55). Selective intra-arterial instillation of radioactive microparticles (56) might be useful in treatments of hepatocellular carcinoma via hepatic artery, since the blood supply of surrounding liver parenchyma comes mainly from the portal vein. ChemoRad nanoparticles were also recently described in the literature as biocompatible lipid–polymer hybrid nanoparticles loaded with both the chemotherapeutic drug (doxetaxel) and radionuclides (111In or 90Y) for chemo- RT of prostate cancer cells (46).
Neutron capture therapy using nanoparticles
Neutron capture therapy (NCT) has been investigated in clinical trials of glioblastoma, malignant melanoma, and head and neck cancer (57-59). Essentially, this modality of RT is based on the increased nuclear interaction cross-section of thermal neutrons (epithermal neutrons slow down to this level after colliding with nitrogen and hydrogen atoms along the way) with boron atoms (isotope B10). The resulting localized nuclear reaction within the tumor creates a high-energy alpha (α2+) particle and a high-energy stable 7Li nucleus, both of which have short path lengths shorter than the diameter of a typical cell, where they cause a dense cluster of ionizations accounting for a high LET. This localized dose delivery combined with the inability of neutron radiation by itself to damage tissue makes boron NCT a promising therapeutic modality. So it gives promising concepts for targeted radiotherapy. Among the many challenges for widespread clinical implementation of NCT, one that can potentially be solved by nanoparticulate formulation of boronated compounds is that of tumor-specific uptake of boron without significant accumulation in normal tissues. This is particularly crucial for boron NCT because the tissue damage is largely confined to the boron-containing tumor cell. Not surprisingly, nanoparticles have been shown to play a role in improving the delivery of boron atoms to cancer cells. Nanoscaled dimensions of boron-capture particles generated via ball milling techniques have been shown to facilitate cellular uptake (60). Significant tumor growth delay was observed by neutron irradiation of boron nanoparticle laden tumors in in vivo studies (61). Liposome formulations (smaller than 100 nm) loaded with boron atoms have demonstrated enhanced tumor uptake leading to tumor growth suppression after NCT (30). More recent research on nanoparticle based BNT includes the design of (I) multifunctional gold nanoparticles decorated with fluorescent dye, boron, and folic acid for targeted delivery to tumor tissue (31); (II) boron nitride nanotubes as theranostic probes for simultaneous MR imaging and NCT (32), similar to that of gadolinium nanoparticles (62,63). Similarly, other nanoparticle based candidates for NCT include dirhenium decacarbonyl [Re2(CO)10] encapsulated in poly-L-lactide (PLLA)-based nanoparticles (64) and holmium-loaded PLLA nanoparticles (65). Lastly, NCT using holmium-containing mesoporous silica nanoparticles demonstrated enhanced survival of mice with ovarian tumors (66). It remains to be seen whether nanopar¬ticle formulations will increase accumulation of neutron-absorbing nonradioactive isotopes within tumors and thereby increase tumor dose without increasing normal tissue dose to realize the promise of enhanced therapeutic gain with NCT in a clinical setting.
Other methods of enhancing radiotherapy using nanoparticles
In addition to the methods noted above to sensitize tumors to RT using nanoparticles, there are some reports in the literature that allude to combination of photodynamic therapy with RT. Photodynamic therapy induces cytotoxicity by generating singlet oxygen species when illuminated with light in presence of a photosensitizer. Its application to cancerous or non-cancerous lesion is confined to superficial areas that light can reach, such as endobronchial, esophageal, bladder, head and neck, oropharyngeal, eye, and skin lesions. An impediment to its wide acceptance in clinic is the nonspecific distribution of photosensitizer in adjacent normal tissues. Nanotechnology has the potential to enhance photodynamic therapy by selectively delivering drug or the photosensitizer itself (67). Scintillation or persistent luminescence nanoparticles with attached photosensitizers have been synthesized such that they can be excited by RT to generate light that, in turn, stimulates the photosensitizer (68). This approach not only enables photodynamic therapy of deep-seated lesions but also enhances radiation dose effect with additional DNA damage from the photosensitizer.
Whereas the above descriptions of the use of nanotechnology have focused on sensitization of tumors to RT, improvement of the therapeutic ratio of RT can be achieved by protection of adjacent normal tissue as well. Given the EPR effect resulting in preferential accumulation of nanoparticles in tumors, it is hard to conceptualize a way to passively accumulate radioprotective nanoparticles in normal tissues. Consequently, the ideal scenario for nanoparticulate radioprotector use is when selective accumulation in normal tissues can be achieved by active targeting or when there is no tumor being radiated simultaneously (such as to mitigate radiation syndromes from accidental radiation exposure of healthy individuals). In a study of melanin-coated silica nanoparticles administered intravenously to melanoma-bearing nude mice, the radioprotective property of melanin resulted in reduction of hematological toxicity without compromising anti-tumor efficacy of subsequent radioim¬munotherapy with 188Re-labeled melanin-binding antibody due to the accumulation of nanoparticles within the bone marrow (69). Amifostine, a free-radical scavenger used for prevention of xerostomia (dry mouth syndrome) from head and neck cancer RT, is effective when administered intravenously. Amifostine nanoparticles produced by polymeric encapsulation (polylactide-co-glycolide) were shown to significantly protect mice from whole body irradiation when administered orally (70). Cerium oxide (CeO2) nanoparticles (71-73) and fullerene nanoparticles (74,75) are also being investigated as potential free radical scavengers to reduce radiation-induced pneumonitis, gut mucosal injury, xerostomia, and radiation-induced dermatitis in animal models.
Conclusions: caveats and outlook for clinical translation
Interest in using nanotechnology to treat cancer has grown explosively as a result of tremendous versatility in nanoparticle design and the potential for surface modifications to enhance their functionality. These properties can be effectively utilized for numerous applications in the diagnosis and treatment of cancer and RT is no exception. Nanoparticles can modify tumor radiation response either as radiosensitizers or radioprotectors themselves or by mediating the delivery of a payload of another radiation modifying material. In addition to these favorable characteristics, nanoparticles may also be detected using a variety of imaging modalities, potentially allowing for quantitative dosimetry and image-guided RT. Potential applications of nanoparticles in radiation oncology are illustrated in Figure 1. While excitement about the impact of nanotechnology on radiation oncology is growing, research in this arena is still in its infancy with most studies confined to proof-of-principle experiments and modeling.
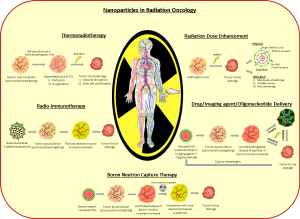
Successful clinical translation of nanoparticles will require investigators to navigate a number of unique challenges in both preclinical evaluation and clinical trial design. The first challenge is to ensure immediate and long-term safety and tolerability in humans. Some particles, such as gold, have years of track record of safe clinical use in other diseases like arthritis. However each new particle will require meticulous preclinical testing and documentation of safety and tolerability thresholds before advancing to early phase clinical trials. As part of this process, the pharmacokinetics, biodistribution and clearance of nanoparticles need to be thoroughly investigated. The functionality of nanoparticles might be limited by nonspecific uptake, assisted by plasma protein opsonization that clears nanoparticles via the reticulo-endothelial system (liver, spleen, lymph nodes). This nonspecific uptake could significantly minimize the circulation half-life of these particles, thus leading to less accumulation in tumors. Ways to minimize nonspecific uptake might include: (I) reducing nanoparticle size; (II) changing the shape of particles (elongated particles are more likely to extravagate from blood vessels since they travel at the periphery of a blood column, rather than spherical particles that travel at the center); (III) changing the surface charge (generally positively charged particles are cleared more rapidly by opsonization while particles with a slightly negative charge keep particles in suspension without clumping and neutral charge minimizes chemical interactions allowing particles to remain in circulation); (IV) using surface modification with a material like polyethylene glycol or dextran to evade macrophages; and (V) inhibiting Kupffer cell activation in the liver. Additionally, the unique operating constraints (type of nanoparticle and the energy source) associated with each of the strategies sets the limitation on the use of these strategies for specific oncologic applications.
Despite the aforesaid limitations, the clinical translation of nanoparticle based strategies in radiation oncology is probably a matter of time because of the prior history of interactions between the physical and life sciences in the field of radiation oncology, the conceptual foundation of both disciplines in the quantitative sciences, and the versatile characteristics of nanoparticles that enable need-based tunability to overcome the roadblocks for specific oncologic applications. Nevertheless, a detailed understanding of the operating constraints and the nano-scale physical and biological underpinnings of nanoparticle-radiation interactions is needed to advance these strategies towards clinical translation.
Acknowledgments
Funding: This work was funded in part by grants from the National Institutes of Health (1R21CA133691-01, 1R01CA132032, 1R01CA155446, and U01CA151886), Department of Defense (PC111832), MD Anderson Institutional Research Grant to SK and NIH-Head and Neck SPORE (P50 CA097007) career development award to PD.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Rao V. L. Papineni, Pataje G.S. Prasanna, Mansoor M. Ahmed) for the series “Nanotechnology in Radiation Research” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.08.10). The series “Nanotechnology in Radiation Research” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roti Roti JL. Introduction: radiosensitization by hyperthermia. Int J Hyperthermia 2004;20:109-14. [PubMed]
- Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol) 2007;19:418-26. [PubMed]
- Song CW, Park HJ, Lee CK, et al. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia 2005;21:761-7. [PubMed]
- Song CW, Shakil A, Osborn JL, et al. Tumour oxygenation is increased by hyperthermia at mild temperatures. 1996. Int J Hyperthermia 2009;25:91-5. [PubMed]
- Lai CY, Kruse DE, Caskey CF, et al. Noninvasive thermometry assisted by a dual-function ultrasound transducer for mild hyperthermia. IEEE Trans Ultrason Ferroelectr Freq Control 2010;57:2671-84. [PubMed]
- Jain PK, Huang X, El-Sayed IH, et al. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc Chem Res 2008;41:1578-86. [PubMed]
- Kennedy LC, Bickford LR, Lewinski NA, et al. A new era for cancer treatment: gold-nanoparticle-mediated thermal therapies. Small 2011;7:169-83. [PubMed]
- Diagaradjane P, Shetty A, Wang JC, et al. Modulation of in vivo tumor radiation response via gold nanoshell-mediated vascular-focused hyperthermia: characterizing an integrated antihypoxic and localized vascular disrupting targeting strategy. Nano Lett 2008;8:1492-500. [PubMed]
- Atkinson RL, Zhang M, Diagaradjane P, et al. Thermal enhancement with optically activated gold nanoshells sensitizes breast cancer stem cells to radiation therapy. Sci Transl Med 2010;2:55ra79 [PubMed]
- Kim J, Oh J, Kang HW, et al. Photothermal response of superparamagnetic iron oxide nanoparticles. Lasers Surg Med 2008;40:415-21. [PubMed]
- Kobayashi T. Cancer hyperthermia using magnetic nanoparticles. Biotechnol J 2011;6:1342-7. [PubMed]
- Rosensweig RE. Heating magnetic fluid with alternating magnetic field. J Magn Magn Mater 2002;252:370-4.
- Jordan A, Wust P, Fähling H, et al. Inductive heating of ferrimagnetic particles and magnetic fluids: physical evaluation of their potential for hyperthermia. 1993. Int J Hyperthermia 2009;25:499-511. [PubMed]
- van Landeghem FK, Maier-Hauff K, Jordan A, et al. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials 2009;30:52-7. [PubMed]
- Johannsen M, Gneveckow U, Taymoorian K, et al. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: results of a prospective phase I trial. Int J Hyperthermia 2007;23:315-23. [PubMed]
- Maier-Hauff K, Rothe R, Scholz R, et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: results of a feasibility study on patients with glioblastoma multiforme. J Neurooncol 2007;81:53-60. [PubMed]
- Wheldon TE, O’Donoghue JA. The radiobiology of targeted radiotherapy. Int J Radiat Biol 1990;58:1-21. [PubMed]
- Pignol JP, Rakovitch E, Beachey D, et al. Clinical significance of atomic inner shell ionization (ISI) and Auger cascade for radiosensitization using IUdR, BUdR, platinum salts, or gadolinium porphyrin compounds. Int J Radiat Oncol Biol Phys 2003;55:1082-91. [PubMed]
- Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev 2013;65:71-9. [PubMed]
- Wong C, Stylianopoulos T, Cui J, et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci U S A 2011;108:2426-31. [PubMed]
- Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J 2005;19:311-30. [PubMed]
- Lévy R, Thanh NT, Doty RC, et al. Rational and combinatorial design of peptide capping ligands for gold nanoparticles. J Am Chem Soc 2004;126:10076-84. [PubMed]
- Bernhardt P, Friedland W, Paretzke HG. The role of atomic inner shell relaxations for photon-induced DNA damage. Radiat Environ Biophys 2004;43:77-84. [PubMed]
- Nikjoo H, Emfietzoglou D, Charlton DE. The Auger effect in physical and biological research. Int J Radiat Biol 2008;84:1011-26. [PubMed]
- Butterworth KT, McMahon SJ, Currell FJ, et al. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale 2012;4:4830-8. [PubMed]
- Cho SH. Estimation of tumour dose enhancement due to gold nanoparticles during typical radiation treatments: a preliminary Monte Carlo study. Phys Med Biol 2005;50:N163-73.
- Jones BL, Krishnan S, Cho SH. Estimation of microscopic dose enhancement factor around gold nanoparticles by Monte Carlo calculations. Med Phys 2010;37:3809-16. [PubMed]
- Carter JD, Cheng NN, Qu Y, et al. Nanoscale energy deposition by X-ray absorbing nanostructures. J Phys Chem B 2007;111:11622-5. [PubMed]
- Leung MK, Chow JC, Chithrani BD, et al. Irradiation of gold nanoparticles by x-rays: Monte Carlo simulation of dose enhancements and the spatial properties of the secondary electrons production. Med Phys 2011;38:624-31. [PubMed]
- Lechtman E, Chattopadhyay N, Cai Z, et al. Implications on clinical scenario of gold nanoparticle radiosensitization in regards to photon energy, nanoparticle size, concentration and location. Phys Med Biol 2011;56:4631-47. [PubMed]
- Lechtman E, Mashouf S, Chattopadhyay N, et al. A Monte Carlo-based model of gold nanoparticle radiosensitization accounting for increased radiobiological effectiveness. Phys Med Biol 2013;58:3075-87. [PubMed]
- Jain S, Coulter JA, Hounsell AR, et al. Cell-specific radiosensitization by gold nanoparticles at megavoltage radiation energies. Int J Radiat Oncol Biol Phys 2011;79:531-9. [PubMed]
- Chithrani DB, Jelveh S, Jalali F, et al. Gold nanoparticles as radiation sensitizers in cancer therapy. Radiat Res 2010;173:719-28. [PubMed]
- Joh DY, Sun L, Stangl M, et al. Selective targeting of brain tumors with gold nanoparticle-induced radiosensitization. PLoS One 2013;8:e62425 [PubMed]
- Geng F, Song K, Xing JZ, et al. Thio-glucose bound gold nanoparticles enhance radio-cytotoxic targeting of ovarian cancer. Nanotechnology 2011;22:285101 [PubMed]
- Butterworth KT, Coulter JA, Jain S, et al. Evaluation of cytotoxicity and radiation enhancement using 1.9 nm gold particles: potential application for cancer therapy. Nanotechnology 2010;21:295101 [PubMed]
- Roa W, Zhang X, Guo L, et al. Gold nanoparticle sensitize radiotherapy of prostate cancer cells by regulation of the cell cycle. Nanotechnology 2009;20:375101 [PubMed]
- Hainfeld JF, Dilmanian FA, Zhong Z, et al. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys Med Biol 2010;55:3045-59. [PubMed]
- Kim JK, Seo SJ, Kim KH, et al. Therapeutic application of metallic nanoparticles combined with particle-induced x-ray emission effect. Nanotechnology 2010;21:425102 [PubMed]
- Kim JK, Seo SJ, Kim HT, et al. Enhanced proton treatment in mouse tumors through proton irradiated nanoradiator effects on metallic nanoparticles. Phys Med Biol 2012;57:8309-23. [PubMed]
- Polf JC, Bronk LF, Driessen WH, et al. Enhanced relative biological effectiveness of proton radiotherapy in tumor cells with internalized gold nanoparticles. Appl Phys Lett 2011;98:193702 [PubMed]
- Chattopadhyay N, Cai Z, Kwon YL, et al. Molecularly targeted gold nanoparticles enhance the radiation response of breast cancer cells and tumor xenografts to X-radiation. Breast Cancer Res Treat 2013;137:81-91. [PubMed]
- Brown JM. Clinical trials of radiosensitizers: what should we expect? Int J Radiat Oncol Biol Phys 1984;10:425-9. [PubMed]
- Williams JA, Yuan X, Dillehay LE, et al. Synthetic, implantable polymers for local delivery of IUdR to experimental human malignant glioma. Int J Radiat Oncol Biol Phys 1998;42:631-9. [PubMed]
- Doiron A, Yapp DT, Olivares M, et al. Tumor radiosensitization by sustained intratumoral release of bromodeoxyuridine. Cancer Res 1999;59:3677-81. [PubMed]
- Wang AZ, Yuet K, Zhang L, et al. ChemoRad nanoparticles: a novel multifunctional nanoparticle platform for targeted delivery of concurrent chemoradiation. Nanomedicine (Lond) 2010;5:361-8. [PubMed]
- Langer R. New methods of drug delivery. Science 1990;249:1527-33. [PubMed]
- Wagner V, Dullaart A, Bock AK, et al. The emerging nanomedicine landscape. Nat Biotechnol 2006;24:1211-7. [PubMed]
- Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med 2012;63:185-98. [PubMed]
- Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano 2009;3:16-20. [PubMed]
- Youan BB. Impact of nanoscience and nanotechnology on controlled drug delivery. Nanomedicine (Lond) 2008;3:401-6. [PubMed]
- Sofou S, Thomas JL, Lin HY, et al. Engineered liposomes for potential alpha-particle therapy of metastatic cancer. J Nucl Med 2004;45:253-60. [PubMed]
- Jonasdottir TJ, Fisher DR, Borrebaek J, et al. First in vivo evaluation of liposome-encapsulated 223Ra as a potential alpha-particle-emitting cancer therapeutic agent. Anticancer Res 2006;26:2841-8. [PubMed]
- Bouchat V, Nuttens VE, Lucas S, et al. Radioimmunotherapy with radioactive nanoparticles: first results of dosimetry for vascularized and necrosed solid tumors. Med Phys 2007;34:4504-13. [PubMed]
- Wu H, Wang J, Wang Z, et al. Apoferritin-templated yttrium phosphate nanoparticle conjugates for radioimmunotherapy of cancers. J Nanosci Nanotechnol 2008;8:2316-22. [PubMed]
- Campbell AM, Bailey IH, Burton MA. Analysis of the distribution of intra-arterial microspheres in human liver following hepatic yttrium-90 microsphere therapy. Phys Med Biol 2000;45:1023-33. [PubMed]
- Hiratsuka J, Fukuda H, Kobayashi T, et al. Human melanoma treated by boron neutron capture therapy: comparison of the clinical response with the predicted response. Radiat Med 1996;14:257-63. [PubMed]
- Kawabata S, Miyatake S, Hiramatsu R, et al. Phase II clinical study of boron neutron capture therapy combined with X-ray radiotherapy/temozolomide in patients with newly diagnosed glioblastoma multiforme--study design and current status report. Appl Radiat Isot 2011;69:1796-9. [PubMed]
- Barth RF, Vicente MG, Harling OK, et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat Oncol 2012;7:146. [PubMed]
- Mortensen MW, Sørensen PG, Björkdahl O, et al. Preparation and characterization of Boron carbide nanoparticles for use as a novel agent in T cell-guided boron neutron capture therapy. Appl Radiat Isot 2006;64:315-24. [PubMed]
- Petersen MS, Petersen CC, Agger R, et al. Boron nanoparticles inhibit tumour growth by boron neutron capture therapy in the murine B16-OVA model. Anticancer Res 2008;28:571-6. [PubMed]
- Arrais A, Botta M, Avedano S, et al. Carbon coated microshells containing nanosized Gd(III) oxidic phases for multiple bio-medical applications. Chem Commun (Camb) 2008;5936-8. [PubMed]
- Fujimoto T, Ichikawa H, Akisue T, et al. Accumulation of MRI contrast agents in malignant fibrous histiocytoma for gadolinium neutron capture therapy. Appl Radiat Isot 2009;67:S355-8. [PubMed]
- Hamoudeh M, Fessi H, Mehier H, et al. Dirhenium decacarbonyl-loaded PLLA nanoparticles: influence of neutron irradiation and preliminary in vivo administration by the TMT technique. Int J Pharm 2008;348:125-36. [PubMed]
- Hamoudeh M, Fessi H, Salim H, et al. Holmium-loaded PLLA nanoparticles for intratumoral radiotherapy via the TMT technique: preparation, characterization, and stability evaluation after neutron irradiation. Drug Dev Ind Pharm 2008;34:796-806. [PubMed]
- Di Pasqua AJ, Yuan H, Chung Y, et al. Neutron-activatable holmium-containing mesoporous silica nanoparticles as a potential radionuclide therapeutic agent for ovarian cancer. J Nucl Med 2013;54:111-6. [PubMed]
- Chatterjee DK, Fong LS, Zhang Y. Nanoparticles in photodynamic therapy: an emerging paradigm. Adv Drug Deliv Rev 2008;60:1627-37. [PubMed]
- Chen W, Zhang J. Using nanoparticles to enable simultaneous radiation and photodynamic therapies for cancer treatment. J Nanosci Nanotechnol 2006;6:1159-66. [PubMed]
- Schweitzer AD, Revskaya E, Chu P, et al. Melanin-covered nanoparticles for protection of bone marrow during radiation therapy of cancer. Int J Radiat Oncol Biol Phys 2010;78:1494-502. [PubMed]
- Pamujula S, Kishore V, Rider B, et al. Radioprotection in mice following oral administration of WR-1065/PLGA nanoparticles. Int J Radiat Biol 2008;84:900-8. [PubMed]
- Colon J, Herrera L, Smith J, et al. Protection from radiation-induced pneumonitis using cerium oxide nanoparticles. Nanomedicine 2009;5:225-31. [PubMed]
- Colon J, Hsieh N, Ferguson A, et al. Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomedicine 2010;6:698-705. [PubMed]
- Madero-Visbal RA, Alvarado BE, Colon JF, et al. Harnessing nanoparticles to improve toxicity after head and neck radiation. Nanomedicine 2012;8:1223-31. [PubMed]
- Daroczi B, Kari G, McAleer MF, et al. In vivo radioprotection by the fullerene nanoparticle DF-1 as assessed in a zebrafish model. Clin Cancer Res 2006;12:7086-91. [PubMed]
- Brown AP, Chung EJ, Urick ME, et al. Evaluation of the fullerene compound DF-1 as a radiation protector. Radiat Oncol 2010;5:34. [PubMed]


