
Tumor microenvironment and nanotherapeutics
Introduction
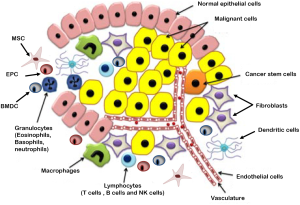
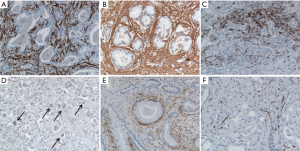
The grand simplification of cancer in research until the end of twentieth century was that it is a disease of the cells. Therefore implying that the disease may be better understood by identifying the genetic changes resulting in altered proteins that disrupt the cell’s communication network causing signals to be garbled, amplified or misdirected, hijacking what was once the normal communication to achieve uncontrolled growth of these genetically altered cells. However, the recent avalanche of information reveals that cancer is actually a dynamic milieu of neoplastic cells and a complex array of non-neoplastic cells that are recruited from the neighboring local or distant host tissue to the tumor microenvironment establishing a favorable niche for the growth of complex tissues that we call tumors (Figure 1) (1-3). These non-neoplastic cells that constitute the tumor microenvironment facilitate tumor development by providing extracellular matrices, cytokines, growth factors, mechanical cues, and vascular networks for nutrient and waste exchange (4). More than 80% of the tumor burden is contributed by derivatives of epithelial tissues, called Carcinomas where the non-neoplastic tumor microenvironment accounts for 30-99% of the tumor mass. Figure 2 elucidates the localization of different cell types that exist in the tumor stroma of histological specimens of Cholangiocarcinoma (5). Thus in the clinical setting it becomes mandatory to understand mechanisms to block the complex crosstalk between cancer cells, their non-neoplastic host cells and the surrounding extracellular matrix that constitute their local environment.
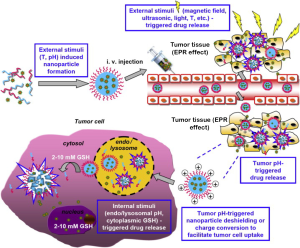
The significant abnormalities in the tumor microenvironment and its cells, such as an acidic pH, altered redox potential, up-regulated proteins and hyperthermia have led to the idea of using stimulus-responsive nanopreparations in antitumor applications (6). This approach is being further extended to design nanopreparations that respond to external stimuli like magnetic field, light and ultrasound for controlled drug release, improved drug internalization and regulation of the intracellular drug fate, resulting in an enhanced tumor targeting and antitumor effect Figure 3 (6-8). Nanotechnology has thus become the emerging field of stimulus-responsive nanoformulations termed “smart drugs” in cancer which (I) utilizes the altered tumor environment to facilitate accumulation of the systemically delivered chemotherapy at the tumor site and (II) enables specific targeting of the tumor and/or tumor microenvironment to achieve tumor growth inhibition (9) and enhanced therapeutic efficacy (10).
Vascular pathophysiology and EPR effect in cancer nanotherapeutics
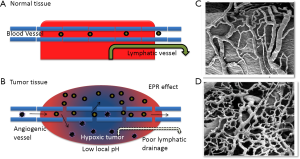
One of the six hallmarks that have been proposed during the development of cancer is sustained angiogenesis (11,12) where the tumors develop their own neovasculature from the existing host microenvironment for nourishment (13). These blood vessels produced within tumors by chronically activated angiogenesis and an unbalanced mix of proangiogenic signals are typically aberrant (14,15). These structural abnormalities result in a leaky vasculature and a poor lymphatic drainage system (16) which causes a differential interstitial pressure (17). The 10-100 nm nanoparticles serving as delivery systems for drugs and small molecules like DNA/RNA utilize this difference in pressure to preferentially accumulate and be retained in tumors unlike the free drugs or small molecules that rapidly undergo renal filtration (18,19). This phenomenon of enhanced permeability and retention (EPR) effect has shown that the retention time of drugs packed in nanoparticles is ten times higher than that of free drugs at the tumor site (20). Hence, this EPR effect attributed to the leaky tumor vasculature is considered as a boon for drug-delivery systems within the nanosize range as described in Figure 4.
Role of altered pH dynamics in the tumor microenvironment in nanotechnology
Tumors contain oxygenated and hypoxic regions (23) and therefore unlike normal cells that derive the bulk of their ATP through mitochondrial oxidative phosphorylation, most cancer cells by what is referred to as ‘Warburg effect’ transition to the less efficient method of glycolysis for energy production, releasing as large amount of lactic acid (24). This method for energy production provides several advantages to the tumor including adaptation to a low oxygen environment and the acidification of the surrounding microenvironment, which promotes tumor invasion and suppresses immune surveillance (25). Nanotechnology utilizes this phenomenon to design pH-sensitive nanoparticles that are stable at a physiologic pH of 7.4, but degraded to release active drug in target tissues in which the pH is less than physiologic values, such as in the acidic environment (6.7-6.9) of tumor cells (26-29). Currently, nearly all successful cancer chemotherapy regimens use a paradigm of multiple drugs given simultaneously. This type of multicomponent chemotherapy has been first demonstrated in nanoscale delivery vehicles by the O’Halloran group where two cytotoxic agents are co-encapsulated into 100 nm liposomes that are stable in serum but release their drug in the low-pH endosome, potentially leading to synergistic drug activities (30). This system is continuously being improvised to encapsulate new drug combinations and covalently attached targeting ligands to direct drugs specifically to the tumor site. Recently it has also been shown that nanoparticles composed of weak polybases when exposed to a pH gradient tend to accumulate preferentially and increase in size/swell when in the low pH regions by a phenomenon termed “pH phoresis”. The tumor tissues provide the required low pH microenvironment where the polybase nanoparticles upon accumulation increase in size and get caught in the fenestrated tumor vasculature, facilitating enhanced delivery of drugs to the tumor site (31).
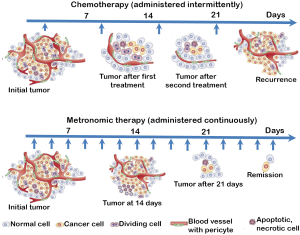
Controlled release in the tumor microenvironment by nanocarriers simulates metronomic therapy
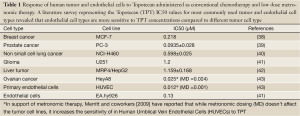
Traditional chemotherapeutic regimens incorporate the “maximum tolerated dose” in the treatment protocols as a standard of care (32). This results in a concomitant overt systemic toxicity which has made it mandatory for the imposition of rest periods between cycles of therapy—a practice that not only involves re-growth of tumor cells, but also growth of selected clones resistant to the therapy reverting to the growth of more malignant metastatic tumors with no therapeutic response. A new philosophy expected to overcome the problems encountered by the conventional treatment regimens that has been introduced by Judah Folkman and Robert Kerbel (33,34) and termed ‘metronomic therapy’ by Douglas Hanahan (35,36) involves a schedule which consists of low doses of chemotherapeutic drugs administered without extended rest periods (Figure 5). The novelty in this concept is in the targeting of the tumor microenvironment, particularly the endothelial cells which are more sensitive to the consistent low dose drug administration than tumor cells, inhibiting tumor angiogenesis eventually resulting in tumor growth inhibition (37). Upon literature survey we found the endothelial cell types to be more sensitive than the tumor cells types to the anticancer drug, Topotecan. Metronomic dosing was more effective in killing the endothelial cells in comparison to the tumor cell types (Table 1). Interestingly, this sustained delivery and controlled release preferentially at the tumor/tumor microenvironment site is a challenge which drives the design of various drug delivery strategies that strive to revolutionize the way drugs exert their actions. Nanosized drug carriers due to their small size, relatively high surface area, influence on biodistribution, their stabilizing effect on therapeutic agents and their ability to make drugs available for intravascular delivery at the tumor site facilitate sustained release of active drug over a period of time simulating the action of metronomic therapy in cancer (44).
Full table
Tumor microenvironment and prodrug therapy
Prodrugs are derivatives of drug molecules that can undergo a transformation by an enzyme, chemical or environmental stimuli to release the active parent drug in vivo (45). A drug which is highly cytotoxic or has a short half-life in circulation may now be administered in an inactive state as a nanoformulation or “prodrug” targeted to the tumor/tumor microenvironment via tumor specific molecules. Upon reaching its destination, the tumor environment facilitates its’ conversion to an active form. This tumor-activated prodrug therapy functions by attacking both the tumor and stroma cells through a “bystander effect” without selectively deleting the target-producing cells, therefore further minimizing resistance and toxicity. Matrix metalloproteinase-2 (MMP-2) is a stroma-derived MMP belonging to the type IV collagenase family playing a critical role in the degradation of basement membranes and the extracellular matrix. The overexpression of matrix metalloproteinase-2 in melanoma has been shown in a number of preclinical as well as clinical investigations. A water-soluble maleimide derivative of doxorubicin, incorporating a matrix metalloproteinase-2-specific peptide sequence developed by Mansour et al. has been shown to have high affinity for the cysteine-34 position of circulating albumin (46). The albumin-bound form of the polymer-drug conjugate was efficiently cleaved by the matrix metalloproteinase-2 enriched in the tumor stroma liberating free doxorubicin. The tumor microenvironment pH and redox potential were other stimuli that triggered drug release triggers at the tumor site (47). Cisplatin, an antiproliferative agent being used in the treatment of cancer since the 1970’s is known for its’ severe side effects that include nephrotoxicity, neurotoxicity (ototoxic), and emetogenic (nausea and vomiting) has been shown by researchers from Lippard’s and Farokhzad’s group at MIT and Harvard respectively for safer and more effective prostate cancer therapy in vivo by the targeted delivery of a cisplatin prodrug. Being highly hydrophilic (water soluble) the half-life of cisplatin is 43 minutes with approximately 1/4th being eliminated within the first 24 hours (90% renal clearance). Encapsulation of the hydrophilic drug in a hydrophobic nanoparticle not only makes it an inactive prodrug but increases it’s half-life in circulation by 5 times and when coated with prostate specific membrane antigen (PSMA) facilitates targeted delivery of cisplatin to prostate cancer cells (48).
Preferential targeting of nanoparticles helps overcome multiple drug resistance (MDR) in cancer
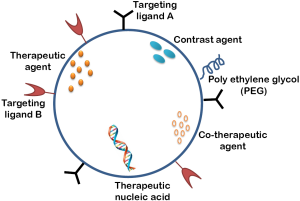
MDR continues to remain a major unresolved challenge in clinical cancer chemotherapy (49). In the clinic, multidrug resistance occurs in over 50% of patients, whose cancer relapses, accounting in large part for the high mortality associated with cancer. Solid tumors exist in an intimate relationship with the surrounding microenvironment, and it is the dynamics of this heterogeneous and ever changing ecosystem that contributes to the initiation and progression of the disease (50-54). In addition to initiating and supporting the tumorigenic process, a permissive microenvironment can also affect the sensitivity of tumor cells to drug treatment (55). The three-dimensional structure of the tumor tissue and the composition and organization of the extracellular matrix (ECM) and stromal components contribute to marked gradients in drug concentration, increased interstitial fluid pressure and metabolic changes, all of which may alter the resistance of tumor cells to cytotoxic agents and radiation (56-61). The tumor microenvironment/architecture has been shown to significantly contribute to the emergence of therapeutic resistance and thus the need for targeting and manipulating this complex symbiotic interplay to overcome MDR (62). The tumor microenvironment induced multidrug resistance occurs via (I) cell-cell and cell-ECM adhesion; (II) cell communication; (III) alterations in mechanosensing; (IV) Phenotypic transitions; and (V) protective quiescence (63). One of the most common mechanisms that has been shown to confer simultaneous resistance to different drugs relies on drug efflux from cancer cells mediated by ATP-binding cassette (ABC) transporters (64). A novel mechanism for the acquisition of drug resistance by tumor endothelial cells (TECs) in a tumour microenvironment to paclitaxel through greater mRNA expression of multidrug resistance 1, which encodes P-glycoprotein, as compared with normal endothelial cells has also been reported. High levels of vascular endothelial growth factor in tumour-conditioned medium were found to be responsible for the upregulated P-glycoprotein expression (65). Nanoparticles with affinity for specific receptors (66) in the tumor/tumor microenvironment when entering the cells, are usually enveloped by endosomes via receptor-mediated endocytosis, thereby bypassing the recognition of P-glycoprotein, one of the prominent ABC transporters mediating multidrug resistance, resulting in the increased intracellular concentration of drugs (67). Human serum albumin encapsulated paclitaxel (also known as Abraxane) is a clinically successful candidate that has been used to target the microenvironment utilizing the high affinity of a 60-kDa glycoprotein, gp60 located on the surface of endothelial cells displays for the albumin-paclitaxel complex (68,69). The albumin-paclitaxel complex when released into the subendothelial space is further enriched by another glycoprotein named SPARC (secreted protein, acidic and rich in cysteine) that binds to albumin with high affinity and has a significant homology to gp60 (70). We have identified Galectin-1, as a tumor vasculature associated protein (71) that is further specifically upregulated in endothelial cells in response to radiation exposure (72). It also serves as a major receptor for the 33 a.a. antiangiogenic peptide Anginex (73) and is thus a promising candidate for radiation enhanced delivery of chemotherapy via Anginex conjugated drug loaded nanoparticles. This multifunctional approach utilizing three modalities viz.: radiation, antiangiogenesis (anginex) and nanosized chemotherapy that is being developed in our laboratory to preferentially target the solid tumor is expected to provide a safer and more effective cancer chemoradiation therapeutic application (72). Multifunctional nanoparticle formulations designed to allow the drug to bypass the efflux of pump transporters or combination delivery and drug efflux modulation simultaneously (74) are now being actively investigated facilitating personalized and tailored cancer treatment (75). These multifunctional nanoparticles are also designed with additional capabilities like targeting ligand and image contrast enhancement that allow the nanoparticle to be used for theranostic imaging where therapy is combined with diagnosis, particularly suitable for disease as complex as cancer. The αvβ3-integrin receptor is predominantly used for targeting vascular endothelial cells, as it is elevated in these cells during angiogenesis. Imaging agents targeting αvβ3 have been developed for MRI (76-79), PET (80) and fluorescence imaging (80-84). A tumor-homing peptide CREKA (Cys-Arg-Glu-Lys-Ala) that forms a distinct meshwork specifically in the tumor stroma synthesized by Simberg and colleagues, has been shown to facilitate accumulation of a CREKA-conjugated superparamagnetic iron oxide (SPIO) nanoparticles in both tumor vessels and stroma, resulting in intravascular clotting in tumor blood vessels. This intravascular clotting further attracts more nanoparticles into the tumor, amplifying the targeting. Such multifunctional targeted-SPIO nanoparticles allow for, (I) high specificity for tumor homing; (II) enhanced magnetic resonance imaging (MRI) in tumor; (III) physical blockade of tumor vessels by local embolism. The clotting caused by CREKA-SPIO nanoparticles in tumor vessels is expected to also improve tumor detection by optical imaging techniques (85). Figure 6 shows a scheme for the design of multifunctional nanoparticles.
Conclusions and perspective
The emerging body of literature reveals that tumors are not merely collections of disorganized tumor cells but maladjusted living entities composed of neoplastic cells and surrounding non-neoplastic cells, termed the tumor microenvironment that are recruited by their neoplastic neighbors to provide essential support for the progressive parasitic growth of the neoplasm. There is compelling evidence to indicate appearance of major structural and functional changes at the interface between tumor cells and adjacent host cells in the cancer microenvironment during the growth and progression of the neoplasm. A better understanding of this intricate ecosystem comprising-the complex nature of tumor cell, host cell interactions, as well as cell-ECM interactions inside a tumor, has led to improved cancer therapies. The emerging field of cancer nanotechnology exploits these unique characteristics of the tumor microenvironment and tumor angiogenesis to design new drug delivery systems that specifically target anti-cancer drugs to tumors. National Cancer Institute has taken recent initiatives to harness the power of nanotechnology to radically change the way cancer is currently being diagnosed, imaged and treated. The nanotechnology market is expected to be worth $1 trillion by 2015 as predicted by the US National Science Foundation. A combination of classical chemo and radiotherapy with anti-inflammatory and antiangiogenic strategies targeting the tumor microenvironment is required to reach long-term efficiency. With the growing number of clinical trials of nanotherapies associated with different targeting strategies and combined with radiotherapy or with conventional chemotherapy, provide adequate evidence of the success of these therapies in the future (86). Nanocarriers, particularly multifunctional systems are thus expected to exist as the main therapeutic arsenal in the near future and play a major role in changing the very foundations of cancer diagnosis, treatment and prevention.
Acknowledgments
Funding: We gratefully acknowledge the research support from National Cancer Institute Grant CA173609 to MU and financial support for postdoctoral traineeship (R25CA153954) to AJ from NCI-CNTC (National Cancer Institute Cancer Nanotechnology Training Center).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Rao V. L. Papineni, Pataje G.S. Prasanna, Mansoor M. Ahmed) for the series “Nanotechnology in Radiation Research” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.08.11). The series “Nanotechnology in Radiation Research” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swartz MA, Iida N, Roberts EW, et al. Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res 2012;72:2473-80. [PubMed]
- Rubin H. Cancer as a dynamic developmental disorder. Cancer Res 1985;45:2935-42. [PubMed]
- Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature 2001;411:375-9. [PubMed]
- Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol 2006;22:287-309. [PubMed]
- Cadamuro M, Morton SD, Strazzabosco M, et al. Unveiling the role of tumor reactive stroma in cholangiocarcinoma: an opportunity for new therapeutic strategies. Transl Gastrointest Cancer 2013;2:130-44.
- Zhu L, Torchilin VP. Stimulus-responsive nanopreparations for tumor targeting. Integr Biol (Camb) 2013;5:96-107. [PubMed]
- Cheng R, Meng F, Deng C, et al. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013;34:3647-57. [PubMed]
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer 2005;5:161-71. [PubMed]
- Cho K, Wang X, Nie S, et al. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res 2008;14:1310-6. [PubMed]
- Mukerjee A, Ranjan AP, Vishwanatha JK. Combinatorial nanoparticles for cancer diagnosis and therapy. Curr Med Chem 2012;19:3714-21. [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [PubMed]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70. [PubMed]
- McAllister SS, Weinberg RA. Tumor-host interactions: a far-reaching relationship. J Clin Oncol 2010;28:4022-8. [PubMed]
- Nagy JA, Chang SH, Shih SC, et al. Heterogeneity of the tumor vasculature. Semin Thromb Hemost 2010;36:321-31. [PubMed]
- Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev 2005;15:102-11. [PubMed]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000;407:249-57. [PubMed]
- Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res 1987;47:3039-51. [PubMed]
- Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev 2008;60:1615-26. [PubMed]
- Greish K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol Biol 2010;624:25-37. [PubMed]
- Puri A, Loomis K, Smith B, et al. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst 2009;26:523-80. [PubMed]
- Konerding MA, Miodonski AJ, Lametschwandtner A. Microvascular corrosion casting in the study of tumor vascularity: a review. Scanning Microsc 1995;9:1233-43; discussion 1243-4. [PubMed]
- McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med 2003;9:713-25. [PubMed]
- Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer 2002;2:38-47. [PubMed]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009;324:1029-33. [PubMed]
- Szala S, Mitrus I, Sochanik A. Can inhibition of angiogenesis and stimulation of immune response be combined into a more effective antitumor therapy? Cancer Immunol Immunother 2010;59:1449-55. [PubMed]
- Yatvin MB, Kreutz W, Horwitz BA, et al. pH-sensitive liposomes: possible clinical implications. Science 1980;210:1253-5. [PubMed]
- Ahn RW, Chen F, Chen H, et al. A novel nanoparticulate formulation of arsenic trioxide with enhanced therapeutic efficacy in a murine model of breast cancer. Clin Cancer Res 2010;16:3607-17. [PubMed]
- Sasidharan A, Chandran P, Menon D, et al. Rapid dissolution of ZnO nanocrystals in acidic cancer microenvironment leading to preferential apoptosis. Nanoscale 2011;3:3657-69. [PubMed]
- Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov 2010;9:615-27. [PubMed]
- Lee SM, O’Halloran TV, Nguyen ST. Polymer-caged nanobins for synergistic cisplatin-doxorubicin combination chemotherapy. J Am Chem Soc 2010;132:17130-8. [PubMed]
- Won YY, Lee H. “pH phoresis”: A new concept that can be used for improving drug delivery to tumor cells. J Control Release 2013;170:396-400. [PubMed]
- Marshall JL. Maximum-tolerated dose, optimum biologic dose, or optimum clinical value: dosing determination of cancer therapies. J Clin Oncol 2012;30:2815-6. [PubMed]
- Browder T, Butterfield CE, Kräling BM, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res 2000;60:1878-86. [PubMed]
- Klement G, Baruchel S, Rak J, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regressionwithout overt toxicity. J Clin Invest 2000;105:R15-24. [PubMed]
- Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest 2000;105:1045-7. [PubMed]
- Pietras K, Hanahan D. A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol 2005;23:939-52. [PubMed]
- Gasparini G. Metronomic scheduling: the future of chemotherapy? Lancet Oncol 2001;2:733-40. [PubMed]
- Timur M, Akbas SH, Ozben T. The effect of Topotecan on oxidative stress in MCF-7 human breast cancer cell line. Acta Biochim Pol 2005;52:897-902. [PubMed]
- Aljuffali IA, Mock JN, Costyn LJ, et al. Enhanced antitumor activity of low-dose continuous administration schedules of topotecan in prostate cancer. Cancer Biol Ther 2011;12:407-20. [PubMed]
- Nagashima S, Soda H, Oka M, et al. BCRP/ABCG2 levels account for the resistance to topoisomerase I inhibitors and reversal effects by gefitinib in non-small cell lung cancer. Cancer Chemother Pharmacol 2006;58:594-600. [PubMed]
- Yang X, Zhang C, Ying M, et al. Antiproliferation in human EA.hy926 endothelial cells and inhibition of VEGF expression in PC-3 cells by topotecan. Pharmazie 2007;62:534-8. [PubMed]
- Tian Q, Zhang J, Chan SY, et al. Topotecan is a substrate for multidrug resistance associated protein 4. Curr Drug Metab 2006;7:105-18. [PubMed]
- Merritt WM, Danes CG, Shahzad MM, et al. Anti-angiogenic properties of metronomic topotecan in ovarian carcinoma. Cancer Biol Ther 2009;8:1596-603. [PubMed]
- Mainardes RM, Silva LP. Drug delivery systems: past, present, and future. Curr Drug Targets 2004;5:449-55. [PubMed]
- Rautio J, Kumpulainen H, Heimbach T, et al. Prodrugs: design and clinical applications. Nat Rev Drug Discov 2008;7:255-70. [PubMed]
- Mansour AM, Drevs J, Esser N, et al. A new approach for the treatment of malignant melanoma: enhanced antitumor efficacy of an albumin-bindingdoxorubicin prodrug that is cleaved by matrix metalloproteinase 2. Cancer Res 2003;63:4062-6. [PubMed]
- Guo X, Szoka FC Jr. Chemical approaches to triggerable lipid vesicles for drug and gene delivery. Acc Chem Res 2003;36:335-41. [PubMed]
- Dhar S, Kolishetti N, Lippard SJ, et al. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc Natl Acad Sci U S A 2011;108:1850-5. [PubMed]
- Ling V. Multidrug resistance: molecular mechanisms and clinical relevance. Cancer Chemother Pharmacol 1997;40:S3-8. [PubMed]
- Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 2004;4:839-49. [PubMed]
- Bhowmick NA, Moses HL. Tumor-stroma interactions. Curr Opin Genet Dev 2005;15:97-101. [PubMed]
- Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem 2007;101:805-15. [PubMed]
- Kopfstein L, Christofori G. Metastasis: cell-autonomous mechanisms versus contributions by the tumor microenvironment. Cell Mol Life Sci 2006;63:449-68. [PubMed]
- Cretu A, Brooks PC. Impact of the non-cellular tumor microenvironment on metastasis: potential therapeutic and imagingopportunities. J Cell Physiol 2007;213:391-402. [PubMed]
- Trédan O, Galmarini CM, Patel K, et al. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 2007;99:1441-54. [PubMed]
- Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci 1999;24:68-72. [PubMed]
- Heldin CH, Rubin K, Pietras K, et al. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer 2004;4:806-13. [PubMed]
- Di Paolo A, Bocci G. Drug distribution in tumors: mechanisms, role in drug resistance, and methods for modification. Curr Oncol Rep 2007;9:109-14. [PubMed]
- Upreti M, Jamshidi-Parsian A, Koonce NA, et al. Tumor-Endothelial Cell Three-dimensional Spheroids: New Aspects to Enhance Radiation and Drug Therapeutics. Transl Oncol 2011;4:365-76. [PubMed]
- Durand RE, Sutherland RM. Effects of intercellular contact on repair of radiation damage. Exp Cell Res 1972;71:75-80. [PubMed]
- Sutherland RM, Eddy HA, Bareham B, et al. Resistance to adriamycin in multicellular spheroids. Int J Radiat Oncol Biol Phys 1979;5:1225-30. [PubMed]
- Ozben T. Mechanisms and strategies to overcome multiple drug resistance in cancer. FEBS Lett 2006;580:2903-9. [PubMed]
- Correia AL, Bissell MJ. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist Updat 2012;15:39-49. [PubMed]
- Szakács G, Paterson JK, Ludwig JA, et al. Targeting multidrug resistance in cancer. Nat Rev Drug Discov 2006;5:219-34. [PubMed]
- Akiyama K, Ohga N, Hida Y, et al. Tumor endothelial cells acquire drug resistance by MDR1 up-regulation via VEGF signaling in tumor microenvironment. Am J Pathol 2012;180:1283-93. [PubMed]
- Peer D, Karp JM, Hong S, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2007;2:751-60. [PubMed]
- Larsen AK, Escargueil AE, Skladanowski A. Resistance mechanisms associated with altered intracellular distribution of anticancer agents. Pharmacol Ther 2000;85:217-29. [PubMed]
- Schnitzer JE. gp60 is an albumin-binding glycoprotein expressed by continuous endothelium involved in albumin transcytosis. Am J Physiol 1992;262:H246-54. [PubMed]
- Tiruppathi C, Song W, Bergenfeldt M, et al. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J Biol Chem 1997;272:25968-75. [PubMed]
- Sage H, Johnson C, Bornstein P. Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J Biol Chem 1984;259:3993-4007. [PubMed]
- Camby I, Le Mercier M, Lefranc F, et al. Galectin-1: a small protein with major functions. Glycobiology 2006;16:137R-157R. [PubMed]
- Upreti M, Jamshidi-Parsian A, Apana S, et al. Radiation-induced galectin-1 by endothelial cells: a promising molecular target for preferential drug delivery to the tumor vasculature. J Mol Med (Berl) 2013;91:497-506. [PubMed]
- Thijssen VL, Postel R, Brandwijk RJ, et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc Natl Acad Sci U S A 2006;103:15975-80. [PubMed]
- Raub TJ. P-glycoprotein recognition of substrates and circumvention through rational drug design. Mol Pharm 2006;3:3-25. [PubMed]
- Mozafari MR, Pardakhty A, Azarmi S, et al. Role of nanocarrier systems in cancer nanotherapy. J Liposome Res 2009;19:310-21. [PubMed]
- Mulder WJ, Strijkers GJ, Habets JW, et al. MR molecular imaging and fluorescence microscopy for identification of activated tumor endothelium using a bimodal lipidic nanoparticle. FASEB J 2005;19:2008-10. [PubMed]
- Sipkins DA, Cheresh DA, Kazemi MR, et al. Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging. Nat Med 1998;4:623-6. [PubMed]
- Winter PM, Caruthers SD, Kassner A, et al. Molecular imaging of angiogenesis in nascent Vx-2 rabbit tumors using a novel alpha(nu)beta3-targeted nanoparticle and 1.5 tesla magnetic resonance imaging. Cancer Res 2003;63:5838-43. [PubMed]
- Zhang C, Jugold M, Woenne EC, et al. Specific targeting of tumor angiogenesis by RGD-conjugated ultrasmall superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner. Cancer Res 2007;67:1555-62. [PubMed]
- Chen X, Park R, Shahinian AH, et al. 18F-labeled RGD peptide: initial evaluation for imaging brain tumor angiogenesis. Nucl Med Biol 2004;31:179-89. [PubMed]
- Achilefu S, Bloch S, Markiewicz MA, et al. Synergistic effects of light-emitting probes and peptides for targeting and monitoring integrin expression. Proc Natl Acad Sci U S A 2005;102:7976-81. [PubMed]
- Chen X, Sievers E, Hou Y, et al. Integrin alpha v beta 3-targeted imaging of lung cancer. Neoplasia 2005;7:271-9. [PubMed]
- Hsu AR, Hou LC, Veeravagu A, et al. In vivo near-infrared fluorescence imaging of integrin alphavbeta3 in an orthotopic glioblastoma model. Mol Imaging Biol 2006;8:315-23. [PubMed]
- Wang W, Wu Q, Pasuelo M, et al. Probing for integrin alpha v beta3 binding of RGD peptides using fluorescence polarization. Bioconjug Chem 2005;16:729-34. [PubMed]
- Simberg D, Duza T, Park JH, et al. Biomimetic amplification of nanoparticle homing to tumors. Proc Natl Acad Sci U S A 2007;104:932-6. [PubMed]
- Ruenraroengsak P, Cook JM, Florence AT. Nanosystem drug targeting: Facing up to complex realities. J Control Release 2010;141:265-76. [PubMed]


