Nanoparticles in radiation therapy: a summary of various approaches to enhance radiosensitization in cancer
Introduction
A major reason for the absence of cure and subsequent tumor relapse is the development of resistance to the therapeutic modality. Radiation therapy along with surgery and chemotherapy are the major therapeutic strategies for cancer treatment. It involves the delivery of high intensity ionizing radiations with high accuracy to the tumor tissue resulting in the death of tumor cells. Radiation therapy has its disadvantages including the possibility of injury to the surrounding normal tissue. Another disadvantage is that some tumor cells are farther away from the site of radiation and hence might receive a lower intensity of the radiation beam. Moreover, the cells can develop resistance to the radiation. Usually the sensitivity of the mitotically active tumor cells is only slightly higher than that of surrounding healthy tissue. Hence the minimum dose of radiation that is sufficient to kill tumor tissue may only injure but not kill the normal tissue. However, due to development of resistance of tumor cells to the dosed radiation results in requirement of elevated doses which eventually leads to death of the healthy tissue.
High-energy ionizing radiations such as gamma rays or X-rays are mainly used to ionize cellular components and/or water. Particulate radiations such as alpha or beta particles or electron, proton or neutron beams are also used in certain specific cases to target the cancer tissue (1,2). Since water is a major component of the cells it is the major target of these ionizing radiations which result in radiation mediated lysis of the molecule. Unlike a chemical lysis, this radiolysis results in the generation of not only charged species but also free radicals such as hydrogen radical H•, hydroxyl radical OH•, Superoxides O2- and charged water species such as H2O+, and H2O+. DNA is the primary target of the ionizing radiations themselves along with the radicals though many other cellular components are also damaged (3). Interaction of free radicals with the membrane structures also causes structural damages resulting in induction of apoptosis. The hydroxyl ion has been reported in multiple studies to be a major source of cellular damage and it is known to induce lipid peroxidation. The interaction with lipid bilayers have also been shown make the cells highly permeable.
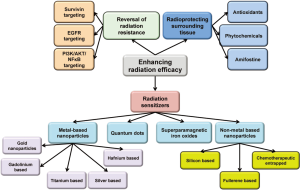
Though great advances have been made in the field of radiation oncology resulting in better focusing and more regulated dosing of the ionizing radiation, some major issues with the therapy still remain. Radiation resistance as well as the inherent flaws of the therapeutic system still makes it a balancing act between its therapeutic advantages and physiological disadvantages. Multiple approaches have been used to enhance its efficacy while reducing the toxicity. The three major approaches that will be discussed in this mini review will be (I) ehancing radiosensitization of tumor tissue; (II) reversal of radiation resistance in tumor tissue; and (III) enhancing radioresistance of the healthy tissue. Approaches used for radiosensitization have been summarized in Figure 1.
Enhancing the efficacy of radiation therapy by radiosensitizers
Radiation sensitization is a process of enhancing the susceptibility of tumor tissues to injury by radiation exposure. Hence, rediosensitizers are therapeutic or otherwise inert agents that enhance the effects of radiation therapy. Over the last few years there has been a considerable increase in interest in the use of formulations to enhance radiotherapeutic effects, especially using metal (mainly gold) based nanoparticles (4). The densely packed metal particles can selectively scatter and/or absorb the high energy gamma/X-ray radiations. This allows for better targeting of cellular components within the tumor tissues allowing for more localized and consolidated damage. These also provide enriched interaction cross-section with the photons from these radiations (5,6). The photoelectron scattering upon the exposure of the surface of the metals to the gamma irradiation is also proposed to be mechanism for enhanced activity. A combination of all these phenomenon results in reduction is the therapeutic radiation dose further limiting the damage to the healthy tissue. The use of nanomaterial radiosensitizers is also called as Nanoparticle Enhanced X-ray Therapy or NEXT (7).
The earliest studies demonstrating enhanced radiation damage of the chromosomal DNA occurred in the mid-1970s when patients undergoing iodine angiography showed enhanced lymphocyte toxicity (8). In vitro studies conducted during the same period also showed similar enhancements in cytotoxic effects of radiation in presence of Iodine (9). This resulted in the development of the concept in which high-Z material when incorporated into cells results in higher efficiency for radiation mediated cellular damage. In separate studies it was demonstrated that cells grown on gold film showed a multifold and significant dose enhancement effect upon irradiation. In other studies, tumor tissues injected with ≈3 µm sized gold nanoparticles showed much reduced growth post irradiation. The problem with these particles was the lack of diffusion in the cancer tissue due to their large size. Thus based on the same principles smaller sized gold nanoparticles have been extensively optimized and utilized in various cancers.
Principles of radiosensitization by metal-based formulations
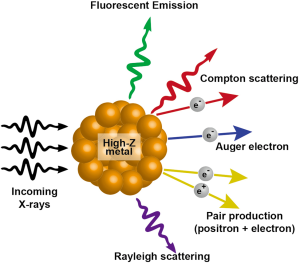
When X-rays hit a metal, there are multiple possibilities of eventual outcome. Among the several emissions that occur, the most relevant to cancer radiotherapy are scattered X-rays/photons, photoelectrons, Compton electrons, Auger electrons and fluorescence photons. The incoming radiation wave imparts its energy to an electron within the atom ejecting it from its orbital with a kinetic energy equivalent of the energy of the wave minus the binding energy of the electron. This kinetic energy of the outgoing electron radiation is what decides the range of the electron within the tissue. This photoelectric effect is decided by (Z/E)3 where E is the energy of the incoming photon and Z is the atomic number of the molecule being targeted. The Auger electrons or fluorescent photons are produced when the ejected electrons are replaced with electrons dropping from the higher orbits and energy is released. The fluorescent photons are low energy but have higher coverage range. The Auger electrons have much shorter range of coverage but can generate much higher ionization density at a localized area. Gold being a high-Z material (Z=79) and very inert to tissue interactions is ideal for photosensitization reactions. The interaction of X-rays with high-Z nanoparticles and the resultant outcomes have been summarized in Figure 2.
Properties of gold nanoparticles
The advantages of gold nanoparticles that make it an ideal material for photosensitization among high Z particles are the following (10):
- Gold being very inert, it is highly biocompatible;
- The gold nanoparticles enhance the effect of the radiation over a large area of tumor thus eliminating the need of the nanoparticles to be delivered to all the cells of the tumor tissue;
- Nanoparticles are known to have low systemic clearance as compared to low molecular contrast agents such as iodine allowing the photosensitizing material enough time to get absorbed into the tumor tissue;
- Nanoparticles are known to be well absorbed into systemic circulations, better permeation into the tumor tissue. This along with lower clearance rate results in the enhanced permeation and retention (EPR) effect;
- By attaching targeting moieties such as antibodies, large number of the gold atoms can be specifically delivered to the tumor tissue as compared to using solutions of iodine. A nanoparticle of 10-15 nm in size contains 50-75 thousand atoms within it resulting in a much higher efficiency of delivery;
- The gold nanoparticles can be varied in size or shapes (such as spheres cube, rods, cones or other 3D structures) based on the delivery requirements of the tumor tissue (such as its size and location) so as to achieve optimum delivery and effect;
- It is much easier to perform overall and tissue specific pharmacokinetic studies with the gold nanoparticles as they are easy to image and quantify. Thus the dose levels can be optimized for best results.
Along with these advantages, there are certain disadvantages associated with the use of gold nanoparticles such as the high cost of material and formulation. Though the EPR effect has its own advantages, the long circulating half-life may not be beneficial when considered at a whole body level. Though as such gold is supposed to be inert, more detailed toxicological profile still needs to be generated. Surface coating using polymeric material has led to better regulation of the pharmacokinetic and targeting properties of the gold nanoparticles (11). The gold nanoparticle itself provides large number of ligand binding sites. The number of binding sites is directly proportional to the size of the nanoparticle. The advantage of these ligand-binding sites is that the chemistry for the attachment is relatively easy, and the surface properties allow for the binding of multiple different types of ligands to the same nanoparticle. Due to the cost associated with the therapy using gold, just modifying the size of the nanoparticles itself to adjust the pharmacokinetic properties of the formulation may not be the best approach. The use of polymeric coating may be a better approach to play around with the size of the formulation. Also the possibility of attaching multiple different ligands allows for the attachment of polymeric materials such as PEG along with other targeting moieties. PEG has been shown in multiple studies to reduce the uptake of nanoparticulate formulations by reticuloendothelial system (12). This allows for prolonged retention of the gold nanoparticles within the circulatory system. As previously mentioned, the efficacy of the metal-based formulation depends upon the energy of the radiation along with the type, amount and location of material within the tissue. Better targeting and pharmacokinetic profile of the nanoparticles will generate much more efficient therapy with reduced adverse effects to surrounding healthy tissue.
Therapeutic uses of gold nanoparticles in radiosensitization
Zheng and colleagues did a proof of principal study for the enhanced radiosensitization effects of gold nanoparticles on DNA damage induced by high energy electrons (13). They used plasmid DNA and bombarded them with 60 keV electrons either alone or in the presence of gold nanoparticles at a ratio of 1:1 or 1:2 DNA to gold nanoparticle. This increased the number of double stranded breaks by a magnitude of about 2.5 fold. The studies suggested that the enhanced effects were due to the production of low energy electrons from the gold particles and that the effects were directly proportional to the number of particles in the proximity of the DNA. Based on similar concepts, one of the first systemic optimization studies was performed by Brun and colleagues, where they further studied parameters such as size (8-92 nm) and molar ratio of the nanoparticles along with the energy of the incident X-rays (14.8-70 keV) (14). In these studies, the best results were achieved when using gold nanoparticles of large size, at high molar concentration and with 50-keV photons. This combination resulted in a 6-fold improvement relative to controls.
Additional optimization studies by Lechtman and group also had very interesting outcomes (15). Based on the results of their studies, they concluded that when using photon energies below the k-edge, auger cascade is dominant and hence small sized nanoparticles need to be located in close proximity of the eventual target sites within the cellular compartments. However, the use of photon sources above the k-edge requires a higher gold concentration in the tumor region but in these cases the size and localization of the nanoparticles is not a significant factor (15). The authors recently also generated a Monte Carlo-based model for prediction of gold nanoparticle radiosensitization, which takes into account the detailed energy deposition at the nanoscale. The claims by these authors though have been disputed by McMahon et al. stating there may be a potential disparity between the theoretical predictions and actual clinical outcomes (16).
The role of size in deciding the eventual sensitization outcome of nanoparticles depends of the balancing act between the effect of size on uptake as well as effect of size on photon production and range. Therefore, increasing the uptake of particles into cells, with larger diameter of the particles may have the most optimal outcome. The use of gold nanoparticles as radiosensitizing agents for low dose rate gamma radiation therapy such as with I-125 brachytherapy seeds has also been recently shown by Ngwa and colleagues (17). They found a 70-130% increase in the therapeutic efficacy in the presence of the nanoparticles. Most of the toxicological responses are due to the gold accumulation and liver toxicity. With the increased interests in the use of gold nanoparticles in cancer therapy, more sensitive detection methods have been developed allowing for more accurate dosimetry (18).
To study the effects of gold nanoparticles in combination with radiotherapy in specified cancers Joh et al. studied the effect of gold nanoparticles in sensitizing glioblastoma cells and tumors to radiation therapy (19). They found that the gold nanoparticles not only enhanced the radiation effects in vitro but also showed significantly higher brain endothelial cell death. The treatment increased the survival rate in mice with orthotopic glioblastoma multiforme tumors. Separate studies by Bobyk and group on mice models of glioma showed a similar increase in efficacy and improved survival rate with gold nanoparticles on 1.9 nm size in combination with low energy radiation therapy (20).
To determine whether gold nanoparticles have higher activity, Xiao et al. coated them with thiolated undecane [S-C(11)H(23)], or with dithiolated diethylenetriaminepentaacetic (DTDTPA) or gadolinium (Gd) DTDTPA chelating agents. The studies using these coated nanoparticles showed attenuated effects as compared to the naked nanoparticles (21). These studies suggested that coatings may considerably diminish the short-range low-energy electrons emitted from gold, leading to a considerable decrease of radiosensitization. However, independent studies conducted by multiple groups using PEG coated gold nanoparticles showed increased therapeutic efficacy of the formulation for radiosensitization (22-24). Various sized nanoparticles have been studied and in each case a concentration dependent increase in efficiency of killing the cancer cells was observed. This increased efficacy was attributed to the EPR benefits of the PEG coating rather than its effects on energy redistribution. Studies in fields other than radiotherapy suggest there may be potential interference of PEG with the photon production by the gold particles. In addition, studies carried out to determine the toxicological effects of PEG coated gold nanoparticles in healthy tissue suggest that there is no enhanced toxicity associated with these coated nanoparticles (25) though these effects may be size dependent (26,27) or concentration dependent (10) or vary based on the administration route (28).
Combination of the gold nanoparticles with other radiosensitizers either by co-administration or by conjugation have also been utilized extensively so as to increase tumor cytotoxicity, while simultaneously minimizing effects on healthy surrounding tissue. Jeong and colleagues used gold nanoparticles as a carrier for delivery of ss-lapachone a novel anticancer agent displaying potent cytotoxicity against cancer cells as well excellent radiosensitizer (29). The combination was shown to have significant enhancements in activity. Further introduction of anti-EGFR antibody as a targeting moiety for cancer further enhanced the effects (29). DNA condensation by avidin has radioprotective effects in cancer (30). Moreover, the DNA:avidin interaction is reversible with biotin. Based on this theory the authors have hypothesized that by using combination of biotin and gold nanoparticles can synergistically make the target DNA more susceptible to radiation induced damage (30). Human Epidermal Growth Factor Receptor-2 (HER-2)-targeted gold nanoparticles have been synthesized by conjugating trastuzumab (Herceptin) to 30 nm gold nanoparticles. Herceptin acts as both a targeting moiety as well as a mono-therapeutic agent. These conjugated nanoparticles were able to increase the cytotoxic effects of radiation by 3.3-fold as compared to radiation alone whereas non-targeted nanoparticles showed only 1.7-fold increase in efficiency (31). Gold nanoparticles have also been studied in combination with other chemotherapeutic agents for potential synergy in activity. A multifold increase in the single and double stranded breaks was observed in DNA of the cells in presence of gold nanoparticles and radiation when they were pretreated with cisplatin (32). Cisplatin binding to guanine was shown to result in better bond dissociation by triggering the formation of transient anions. They further went on to study the various stoichiometric combinations of gold nanoparticles and cisplatin on their eventual radiosensitization (33). Binding of a single cisplatin molecule to the nanoparticle resulted in a 3-fold increase in activity whereas combination of 2 cisplatin molecules with a gold nanoparticle resulted in up to 7.5 times more double stranded breaks.
Other metal based based radiosensitizers
Gadolinium was identified as another new class of radiation sensitizers that were also very practical because they could also be easily viewed in vivo through the use of magnetic resonance imaging. It is known to generate long-lived pi-radical cations upon exposure to hydrated electrons. Its effectiveness was studied in vitro in HT-29 cells and also in a murine mammary carcinoma model and was found to be effective in both the cases (34). The effectiveness of the material as such has been subject of debate (35). Gadolinium neutron-capture therapy (NCT) is a therapeutic strategy for cancer, which utilizes the “Gadolinium neutron capture reaction” induced by thermal neutron irradiation. This reaction results in emission of long range gamma rays, internal conversion electrons, X-rays and Auger electrons with large total kinetic energy. The effectiveness of this therapy for cancer was evaluated using chitosan nanoparticles loaded with Gadolinium-157. Mice with subcutaneous melanoma were injected with this formulation intratumorally and then thermal neutron irradiation was performed. Mice treated with the nanoparticle showed much better therapeutic response as compared to those that were dosed with just the gadolinium solution (36). Recently a detailed study was undertaken using 5 nm size gadolinium based nanoparticle on head and neck squamous cell carcinoma cells. These particles consisted of a core of gadolinium oxide, a shell of polysiloxane and were functionalized by diethylenetriaminepentaacetic acid (DTPA). These formulations were found to possess efficient in vitro radiosensitizing properties at energy of 660 keV (37,38).
Titanium dioxide has also been shown to be useful for killing cancer cells using photocatalytic chemistry (39). Its mechanism of action involves generating reactive oxygen species upon photoexcitation by UV radiation. Again, due to limited penetration depth of UV rays, the material is less effective for deep-rooted tissues. To make them more susceptible to X-ray based stimulation titanium nanoparticles were formed containing gadolinium and further optimized with other rare earth metals. The activation of these nanoparticles by X-rays resulted in the formation of ROS, which resulted in enhanced photosensitization effects in vivo (40). Since elongated organic nanoparticles internalize into cells more effectively than their spherical counterparts of similar volume, titanium dioxide nanotubes were formulated and tested for their radiosensitization effects of glioblastoma (41). TiO2 nanotubes were found to be effective radiosensitizers in SNB-19 and U87MG cells by enhancing the DNA damage and retarding the DNA repair (42). Various other techniques have also been utilized to enhance the radiosensitizing effects of TiO2 nanoparticles such as special dye coating (43) or entrapment of other DNA intercalating chemotherapeutic agents such as doxorubicin (44). Pre-photoactivated TiO2 nanoparticles have also been shown to possess enhance cytotoxic effects in HepG2 cells by induction of double stranded breaks (45).
Silver nanoparticles also have radiosensitizing properties, similar to that seen with gold nanoparticles (46). Silver nanoparticles utilize similar mechanisms of action for radiosensitization effects like other high Z-number atoms. They are more cost effective than gold nanoparticles but relatively less biocompatible (47). Silver nanoparticles have been utilized alone (48) or in combination of other metal oxides such as Fe3O4 for radiation therapy in cancer. Silver nanoparticles of nonconventional shape have also been created and studied for their effectiveness in cancer. Chitosan-coated triangular silver nanoparticles have been formulated and have been shown to possess better radiosensitizing activity when compared to conventional PEG coated gold nanoparticles on human non-small lung cancer cells (49). Silver nanoparticles with multiple different coatings have also been shown to possess additive anticancer properties when combined with IR radiations in Glioma cell lines (50).
Nanoparticles have been made using other rare earth metals and high-Z elements such as using hafnium oxide (HfO2). HfO2 has been shown to possess photo-luminescent properties (51,52). They cause thermal induced stress damage to cellular components. Based on these properties, HfO2 nanoparticles have also been tried and tested for their effects on radiosensitization in HCT116 cells in vitro and in in vivo xenograft mice models. The studies showed a good biocompatibility, biodistribution as well as significant radiosensitization using these nanoparticles (53).
Quantum dots in radiosensitization
Quantum dots discovered in the early 1980s are nanocrystals made of semiconductor materials that display quantum mechanical properties due to their small size. Their semiconductor properties are less than those displayed by bulk semiconductors. Quantum dots made from CaF, LaF, ZnS or ZnO [164] have been suggested for use as radiosensitizers (54,55). Development of photosensitizing quantum dots has been a very active area of interest (56). The mechanism of action for these is based on the principle of generation of radicals upon absorption of visible light by the quantum dots. Since these light waves have much less toxicity as compared X-rays or gamma rays, the overall adverse effects of the therapy are greatly reduced. The major disadvantage of this approach is that light waves within the visible spectrum have very little penetration depth and hence the therapies designed utilizing these mechanisms will be suitable only for superficial cancers (57,58).
Superparamagnetic iron oxide nanoparticles as radiosensitizers
Superparamagnetic iron oxide nanoparticles are mainly made up of either magnetite (Fe3O4) or magnemite (γ Fe2O3). These are especially useful for their superparamagnetic properties, which allow them to be directed and localized to a particular organ by using external magnetic force (59). These are also known to be highly biocompatible with negligible toxicity to healthy tissues allowing for usage in therapy (60). These nanoparticles can produce cytotoxic effects due to the production of ROS such as hydrogen peroxide, hydroxyl radical, hydroperoxyl radical and superoxide anion resulting in DNA and other cell organelle damage. The superparamagnetic iron oxide has been shown to enhance the radiation induced DNA damage by catalyzing the ROS production by these ionizing radiation resulting much stronger oxidative stress (61). This process was tested on MCF-7 cells by enhancing the impact of X-rays on the ROS generation for about 240% (62). Further these nanoparticles have also been used as synergistic carriers for other chemotherapeutic agents such as cisplatin (63,64) and genetic material. Superparamagnetic chitosan iron oxide nanoparticles carrying human Adenovirus type 5 early region 1A (E1A) gene were used to enhance the radiosensitivity of cervical cancer. The gene is known to reduce the expression of HER-2 and increase the expression in p53 both of which are known to play a role in regulating radioresistance in cancer (65). The combinations of genetic therapy with increased oxidative stress by iron oxide nanoparticles further enhance the radiosensitivity of human cervical cancer in xenograft mice (66). The iron superoxides have also been used in composition with other metal-based radiosensitizers such as silver. A multifunctional nanocomposite was generated using Fe3O4/Ag and conjugated with to an epidermal growth factor receptor-specific antibody (C225) (67). This composite can act as a diagnostic tool through MRI as well as a radiosensitizer. It was found to sensitize nasopharyngeal carcinoma cell lines to radiation therapy in a dose dependent manner (67).
Non-metal based radiosensitizers
Silica has been used as a carrier or coating material in nanoparticles containing heavy metals for radiosensitization such as gold (68,69), FeO4 (70) or multicomponent cores (71-73). Moreover, nanoparticles made of silica alone have also been tested for their potential role in radiosensitization. In a recent study by Klein and colleagues, the ultrasmall uncapped and aminosilanized oxidized silicon nanoparticles were tested for their radiosensitization effects in breast cancer (MCF-7) and mouse fibroblast cells (3T3) exposed to X-rays of 3 Gy (74). Though the simple nanoparticles did not display any significant increase in ROS production upon exposure to the X-rays, the aminosilanized oxidized silicon nanoparticles exhibited significantly higher ROS production. These were shown to reach the mitochondria and cause oxidative stress damage within the organelle. Though the oxidized nanoparticles displayed increased ROS activity in both the cancer cells and the normal cells, the effects were significantly higher in the cancer cells. This indicates the relative safety of the therapeutic.
C60 is a fullerene, identified in the early 1990s, with unique globular structure consisting of 32 different member rings and containing a total of 60 carbon atoms. Fullerene C60 possesses potent anti-cancer activities and induces certain markers of autophagy in cancer cells (75). It has significant toxicity to normal tissues, which limits its use as therapeutic (76). Nanocrystals of underivatized fullerene C60 (Nano-C60) have been used in concentrations that are non-toxic to normal cells to study their effects on radiosensitization. B16 and SMMU-7721 cell lines were tested with Nano-C60 and γ-radiation and were found to show enhanced membrane damage and induced apoptotic cell death (77). Nano-C60 has also been shown to possess chemosensitizing activity and hence can serve as a potential adjuvant therapeutic in cancer (75).
Polymeric nanoparticles have also been formulated using various chemotherapeutic agents either alone or in combination to serve as radiosensitizers. Paclitaxel is a potent chemotherapeutic agent that is also known to be a cell cycle specific radiosensitizer (78). This is because it arrests cell cycle progression at G2/M, a stage in which the cells are most susceptible to radiation induced damage. Similarly Etanidazole is a nitroimidazole hypoxic radiosensitizer. The studies were performed with PLGA nanoparticles of the drugs either alone or both together to test for their potential radiosensitizing effects (79). Both the individual drugs and their combination enhanced the susceptibility of the cells to the radiation. The prolonged release of the drug from the formulation allowed radiosensitization of hypoxic cells, which are generally more resistant to radiation induced injury. The combination was found to be more effective than the individual drugs. Genexol-PM, a clinically approved formulation of paclitaxel, was studied as a radiosensitizer using non-small cell lung cancer mouse xenograft models. Again, this formulation was found to be both a better radiosensitizer than the normal drug (with effective concentration half of that of the free drug) as well as a safer therapeutic with much reduced exposure of the drug to the health lung tissue (80).
Other chemotherapeutic agents such as doxorubicin have also been used as radiosensitizers. A nanomiceller composite formulation of doxorubicin displayed significantly enhanced radiation sensitivity in multicellular spheroid of A549 lung cancer cell line (81). The formulation cells treated showed significantly higher radio toxicity as compared to cells treated with drug alone. Biodegradable lipid polymer nanoparticles have also been made using docetaxel as the entrapped drug and targeted to cancer tissue using folate. The studies showed that the targeted nanoparticles showed better radiosensitizing properties as compared to drug alone or unmodified nanoparticles. The studies also showed that the radiosensitizing effects using nanoparticulate formulations depend significantly on the time gap between the dosing of the formulation and the radiation (82).
Enhanced efficacy of radiation therapy by radioprotection of surrounding healthy tissue
Since the major targets of radiation therapy are water and DNA, and these are also present in healthy tissue making them susceptible to injury and significant damage if the energy waves are not properly directed to the targeted tissue. The efficacy of the radiation therapy can be increased and its adverse effects decreased if somehow the surrounding healthy tissue can either be protected from this damage or made less radiation sensitive. Molecules with potential radioprotective effects have been an area of interest for scientists since the World War II. Molecules such as amino acid cysteine have also been known to have radiation protective effects. Studies performed in rats showed that the animals dosed with cysteine were able to withstand normally lethal doses of X-rays (83) and showed much less damage to essential organs (84). Certain natural compounds such as curcumin have been shown to exert a dual mode of action after irradiation depending on its dose (85). It protects the cells against the damaging effects of radiation by reducing oxidative stress and inhibiting transcription of genes related to oxidative stress and inflammatory responses at lower doses in healthy cells. Its radiosensitizing effects in cancer cells maybe due to the upregulation of genes responsible for cell death. Antioxidants have also been shown extensively to be radioprotective especially from the reactive oxygen species induced damage (86).
Amifostine is an adjuvant used in cancer chemotherapy to reduce the incidence of neutropenia-related fever and infection induced by DNA-binding chemotherapeutic agents. It has been studied since late 80s for its potential use as a protectant against radiation induced DNA damage (87,88). Orally administered Amifostine did not show any significant radioprotective activity (89). To remedy this, polymeric nanoparticles of Amifostine were prepared that have revealed to have significant radiation desensitizing effects when administered orally (89). Nanostructural combination of Amifostine and fullerenol C60 has also been shown to possess radioprotective effects in both mammalian cells as well as rats undergoing radiation exposure (90,91). Fullerenol C60 alone also has been shown to diminish the radiosensitivity in single cellular eukaryotes as well as in zebra fish models (92). Amifostine has also been found to restore transcriptional activity of p53 enhancing the apoptotic responses to radiation (93).
Neuroprotective agent citicoline when delivered in the form of transferrin coupled liposomes has been shown to possess protective effects in human ovarian adenocarcinoma cells exposed to radiation but not as much in endothelial cells. Thus though the drug formulation has radiprotective effects, its usefulness in increasing the efficacy of radiation therapy is questionable (94). Cerium oxide nanoparticles act as free radical scavengers by changing the charge state on their surface. Thus they help in protecting the cells from free radical damage caused by radiation. They have been shown to increase the longevity of cells by reducing hydrogen peroxide and ultraviolet radiation induced injury (95). Nitroxide Tempol has also been shown to impart radioprotection of the salivary glands in C3H mice (96).
Enhanced efficacy of radiation therapy by reversal of radiation resistance
There are multiple biological pathways that get activated in cancer that make them either inherently resistant to radiation therapy or acquire resistance upon exposure to radiation. These pathways are especially active in cancer stem cells which are normally quiescent cells within the tumor responsible for maintaining and regenerating of a tumor after therapeutic intervention (97,98). Various drugs and treatment strategies are being designed so as to target these specific pathways making the cancer cells more susceptible to radiation therapy. Survivin is one such target protein which is overexpressed in most human tumors, but its levels are barely detectable in normal tissues (99). It is a regulator of cell division, apoptosis, cellular stress response, and also in the regulation of cell migration and metastasis. Increased survivin expression has been directly linked to acquired resistance to chemotherapeutic agents as well as radiation therapy (100). Though novel small molecular therapies are being worked upon (101,102), most of the therapies currently designed for attacking surviving involve macromolecular approaches such as the use of siRNA or peptides which suffer from multiple drug delivery issues (103,104). To overcome these delivery issues human serum albumin-based nanoparticulate carrier system for plasmid-mediated RNA interference (RNAi) have been designed and tested for their efficacy in reversing surviving mediated radioresistance. Gaca and colleagues tested 220 nm sized nanoformulations for their effects of inhibition of surviving expression and its overall effects on radiosensitization. The results were found to be promising with up to 50% decrease in surviving expression as well as a significant increase in radiation susceptibility of SW480 colorectal cancer cells (105). Survivin siRNA cross-linked iron oxide nanoparticles (CLIO)-Cy5.5 have also been designed which can be better targeted using magnetic fields, but their efficacy in reversing radiation resistance has not yet been tested. Dual targeting of survivin and X-linked IAP (XIAP) by using siRNA was found to be even more effective in reversing the radiation resistance of the colorectal cancer cells. Hence the use of nanotechnology and siRNA in targeting surviving can be a productive approach in the future.
Another major target for reversal of radiation resistance is the epidermal growth factor receptor (EGFR). It is known to be a protooncogene that regulates multiple cellular processes, such as proliferation, differentiation, survival, blood vessel formation, and DNA repair (106,107). EGFR has been shown to be over expressed in multiple cancer types such as squamous cell carcinoma of the head and neck (108). Anti EGFR treatments have been shown to increase therapeutic activity of radiation therapy. Along with the antitumor activity of anti EGFR treatment the combination of radiation further results in strong synergy (109). To test this synergy PLGA nanoparticle encapsulated antisense EGFR oligonucleotides were combined with radiotherapy and the relative radiosensitivity of the SCCVII squamous cells was tested. The results showed that antisense EGFR nanoparticles enhanced radiosensitivity by inhibition of EGFR-mediated mechanisms of radioresistance (110). This is very useful as both the therapies complement each other and cell death can be achieved in cells that are resistant to either EGFR therapy or to radiation.
As mentioned earlier, curcumin has been shown to possess both radiosensitizing as well as radioprotective effects based on the cell types and concentrations. Curcumin is also known to act on multiple essential pathways in cancer responsible for radiation resistance. Inhibition of PI3K/AKT-NF-κB pathway with curcumin has been shown to enhance the radiation-induced apoptosis in human Burkitt’s lymphoma (111). It has also been known to interact with Sonic Hedgehog signaling pathway to elicit its radiosensitizing effects (112). Hence a targeted PLGA nanoparticle formulation of curcumin was developed and tested for its effects on chemotherapeutic and radioresistant effects on cisplatin resistant ovarian cancer cell lines. Other extracts such as raspberry extract and neem leaf extract have also been shown to have increased radiosensitization effects (77). Hence, there is a lot of scope in developing formulations with ingredients from natural products for potential radiosensitization activity.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Rao V. L. Papineni, Pataje G.S. Prasanna, Mansoor M. Ahmed) for the series “Nanotechnology in Radiation Research” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.08.06). The series “Nanotechnology in Radiation Research” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hogle WP. The state of the art in radiation therapy. Semin Oncol Nurs 2006;22:212-20. [PubMed]
- Mallick I, Waldron JN. Radiation therapy for head and neck cancers. Semin Oncol Nurs 2009;25:193-202. [PubMed]
- Halliwell B, Aruoma OI. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett 1991;281:9-19. [PubMed]
- Herold DM, Das IJ, Stobbe CC, et al. Gold microspheres: a selective technique for producing biologically effective dose enhancement. Int J Radiat Biol 2000;76:1357-64. [PubMed]
- Park YS, Liz-Marzán LM, Kasuya A, et al. X-ray absorption of gold nanoparticles with thin silica shell. J Nanosci Nanotechnol 2006;6:3503-6. [PubMed]
- Carter JD, Cheng NN, Qu Y, et al. Nanoscale energy deposition by X-ray absorbing nanostructures. J Phys Chem B 2007;111:11622-5. [PubMed]
- Praetorius NP, Mandal TK. Engineered nanoparticles in cancer therapy. Recent Pat Drug Deliv Formul 2007;1:37-51. [PubMed]
- Adams FH, Norman A, Mello RS, et al. Effect of radiation and contrast media on chromosomes. Preliminary report. Radiology 1977;124:823-6. [PubMed]
- Matsudaira H, Ueno AM, Furuno I. Iodine contrast medium sensitizes cultured mammalian cells to X rays but not to gamma rays. Radiat Res 1980;84:144-8. [PubMed]
- Jeremic B, Aguerri AR, Filipovic N. Radiosensitization by gold nanoparticles. Clin Transl Oncol 2013;15:593-601. [PubMed]
- Larson TA, Joshi PP, Sokolov K. Preventing protein adsorption and macrophage uptake of gold nanoparticles via a hydrophobic shield. ACS Nano 2012;6:9182-90. [PubMed]
- Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol Res 2010;62:90-9. [PubMed]
- Zheng Y, Hunting DJ, Ayotte P, et al. Radiosensitization of DNA by gold nanoparticles irradiated with high-energy electrons. Radiat Res 2008;169:19-27. [PubMed]
- Brun E, Sanche L, Sicard-Roselli C. Parameters governing gold nanoparticle X-ray radiosensitization of DNA in solution. Colloids Surf B Biointerfaces 2009;72:128-34. [PubMed]
- Lechtman E, Chattopadhyay N, Cai Z, et al. Implications on clinical scenario of gold nanoparticle radiosensitization in regards to photon energy, nanoparticle size, concentration and location. Phys Med Biol 2011;56:4631-47. [PubMed]
- McMahon SJ, Prise KM, Currell FJ. Comment on ‘implications on clinical scenario of gold nanoparticle radiosensitization in regard to photon energy, nanoparticle size, concentration and location’. Phys Med Biol 2012;57:287-90; discussion 291-5. [PubMed]
- Ngwa W, Korideck H, Kassis AI, et al. In vitro radiosensitization by gold nanoparticles during continuous low-dose-rate gamma irradiation with I-125 brachytherapy seeds. Nanomedicine 2013;9:25-7. [PubMed]
- Alqathami M, Blencowe A, Yeo UJ, et al. Novel multicompartment 3-dimensional radiochromic radiation dosimeters for nanoparticle-enhanced radiation therapy dosimetry. Int J Radiat Oncol Biol Phys 2012;84:e549-55. [PubMed]
- Joh DY, Sun L, Stangl M, et al. Selective targeting of brain tumors with gold nanoparticle-induced radiosensitization. PLoS One 2013;8:e62425 [PubMed]
- Bobyk L, Edouard M, Deman P, et al. Photoactivation of gold nanoparticles for glioma treatment. Nanomedicine 2013; [Epub ahead of print]. [PubMed]
- Xiao F, Zheng Y, Cloutier P, et al. On the role of low-energy electrons in the radiosensitization of DNA by gold nanoparticles. Nanotechnology 2011;22:465101 [PubMed]
- Liu CJ, Wang CH, Chien CC, et al. Enhanced x-ray irradiation-induced cancer cell damage by gold nanoparticles treated by a new synthesis method of polyethylene glycol modification. Nanotechnology 2008;19:295104 [PubMed]
- Liu CJ, Wang CH, Chen ST, et al. Enhancement of cell radiation sensitivity by pegylated gold nanoparticles. Phys Med Biol 2010;55:931-45. [PubMed]
- Zhang XD, Wu D, Shen X, et al. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials 2012;33:6408-19. [PubMed]
- Cho WS, Kim S, Han BS, et al. Comparison of gene expression profiles in mice liver following intravenous injection of 4 and 100 nm-sized PEG-coated gold nanoparticles. Toxicol Lett 2009;191:96-102. [PubMed]
- Zhang XD, Wu D, Shen X, et al. Size-dependent in vivo toxicity of PEG-coated gold nanoparticles. Int J Nanomedicine 2011;6:2071-81. [PubMed]
- Coulter JA, Jain S, Butterworth KT, et al. Cell type-dependent uptake, localization, and cytotoxicity of 1.9 nm gold nanoparticles. Int J Nanomedicine 2012;7:2673-85. [PubMed]
- Zhang XD, Wu HY, Wu D, et al. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int J Nanomedicine 2010;5:771-81. [PubMed]
- Jeong SY, Park SJ, Yoon SM, et al. Systemic delivery and preclinical evaluation of Au nanoparticle containing beta-lapachone for radiosensitization. J Control Release 2009;139:239-45. [PubMed]
- Riva P, Franceschi G, Riva N, et al. Role of nuclear medicine in the treatment of malignant gliomas: the locoregional radioimmunotherapy approach. Eur J Nucl Med 2000;27:601-9. [PubMed]
- Chattopadhyay N, Cai Z, Kwon YL, et al. Molecularly targeted gold nanoparticles enhance the radiation response of breast cancer cells and tumor xenografts to X-radiation. Breast Cancer Res Treat 2013;137:81-91. [PubMed]
- Zheng Y, Hunting DJ, Ayotte P, et al. Role of secondary low-energy electrons in the concomitant chemoradiation therapy of cancer. Phys Rev Lett 2008;100:198101 [PubMed]
- Zheng Y, Sanche L. Gold nanoparticles enhance DNA damage induced by anti-cancer drugs and radiation. Radiat Res 2009;172:114-9. [PubMed]
- Young SW, Qing F, Harriman A, et al. Gadolinium(III) texaphyrin: a tumor selective radiation sensitizer that is detectable by MRI. Proc Natl Acad Sci U S A 1996;93:6610-5. [PubMed]
- Bernhard EJ, Mitchell JB, Deen D, et al. Re-evaluating gadolinium(III) texaphyrin as a radiosensitizing agent. Cancer Res 2000;60:86-91. [PubMed]
- Tokumitsu H, Hiratsuka J, Sakurai Y, et al. Gadolinium neutron-capture therapy using novel gadopentetic acid-chitosan complex nanoparticles: in vivo growth suppression of experimental melanoma solid tumor. Cancer Lett 2000;150:177-82. [PubMed]
- Mowat P, Mignot A, Rima W, et al. In vitro radiosensitizing effects of ultrasmall gadolinium based particles on tumour cells. J Nanosci Nanotechnol 2011;11:7833-9. [PubMed]
- Rima W, Sancey L, Aloy MT, et al. Internalization pathways into cancer cells of gadolinium-based radiosensitizing nanoparticles. Biomaterials 2013;34:181-95. [PubMed]
- Sotter E, Vilanova X, Llobet E, et al. Niobium-doped titania nanopowders for gas sensor applications. J Optoelectron Adv Mater 2005;7:1395-8.
- Townley HE, Kim J, Dobson PJ. In vivo demonstration of enhanced radiotherapy using rare earth doped titania nanoparticles. Nanoscale 2012;4:5043-50. [PubMed]
- Gratton SE, Ropp PA, Pohlhaus PD, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A 2008;105:11613-8. [PubMed]
- Mirjolet C, Papa AL, Créhange G, et al. The radiosensitization effect of titanate nanotubes as a new tool in radiation therapy for glioblastoma: A proof-of-concept. Radiother Oncol 2013;108:136-42. [PubMed]
- Blatnik J, Luebke L, Simonet S, et al. Dye surface coating enables visible light activation of TiO2 nanoparticles leading to degradation of neighboring biological structures. Microsc Microanal 2012;18:134-42. [PubMed]
- Hong C, An S, Son M, et al. In-vitro cell tests using doxorubicin-loaded polymeric TiO2 nanotubes used for cancer photothermotherapy. Anticancer Drugs 2012;23:553-60. [PubMed]
- Petković J, Küzma T, Rade K, et al. Pre-irradiation of anatase TiO2 particles with UV enhances their cytotoxic and genotoxic potential in human hepatoma HepG2 cells. J Hazard Mater 2011;196:145-52. [PubMed]
- Ma J, Xu R, Sun J, et al. Nanoparticle surface and nanocore properties determine the effect on radiosensitivity of cancer cells upon ionizing radiation treatment. J Nanosci Nanotechnol 2013;13:1472-5. [PubMed]
- Coulter JA, Hyland WB, Nicol J, et al. Radiosensitising nanoparticles as novel cancer therapeutics - pipe dream or realistic prospect? Clin Oncol (R Coll Radiol) 2013;25:593-603. [PubMed]
- Zheng Q, Yang H, Wei J, et al. The role and mechanisms of nanoparticles to enhance radiosensitivity in hepatocellular cell. Biomed Pharmacother 2013; [Epub ahead of print]. [PubMed]
- Boca SC, Potara M, Gabudean AM, et al. Chitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly effective photothermal transducers for in vitro cancer cell therapy. Cancer Lett 2011;311:131-40. [PubMed]
- Xu R, Ma J, Sun X, et al. Ag nanoparticles sensitize IR-induced killing of cancer cells. Cell Res 2009;19:1031-4. [PubMed]
- Brezesinski T, Smarsly B, Iimura K, et al. Self-assembly and crystallization behavior of mesoporous, crystalline HfO2 thin films: a model system for the generation of mesostructured transition-metal oxides. Small 2005;1:889-98. [PubMed]
- Mendoza JG, Frutis MA, Flores GA, et al. Synthesis and characterization of hafnium oxide films for thermo and photoluminescence applications. Appl Radiat Isot 2010;68:696-9. [PubMed]
- Maggiorella L, Barouch G, Devaux C, et al. Nanoscale radiotherapy with hafnium oxide nanoparticles. Future Oncol 2012;8:1167-81. [PubMed]
- Juzenas P, Chen W, Sun YP, et al. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv Drug Deliv Rev 2008;60:1600-14. [PubMed]
- Wang YH, Chen Z, Zhou XQ. Synthesis and photoluminescence of ZnS quantum dots. J Nanosci Nanotechnol 2008;8:1312-5. [PubMed]
- Bakalova R, Ohba H, Zhelev Z, et al. Quantum dots as photosensitizers? Nat Biotechnol 2004;22:1360-1. [PubMed]
- Shao L, Gao Y, Yan F. Semiconductor quantum dots for biomedicial applications. Sensors (Basel) 2011;11:11736-51. [PubMed]
- Chatterjee DK, Fong LS, Zhang Y. Nanoparticles in photodynamic therapy: an emerging paradigm. Adv Drug Deliv Rev 2008;60:1627-37. [PubMed]
- Wadajkar AS, Menon JU, Kadapure T, et al. Design and Application of Magnetic-based Theranostic Nanoparticle Systems. Recent Pat Biomed Eng 2013;6:47-57. [PubMed]
- Mikhaylov G, Vasiljeva O. Promising approaches in using magnetic nanoparticles in oncology. Biol Chem 2011;392:955-60. [PubMed]
- Huang G, Chen H, Dong Y, et al. Superparamagnetic iron oxide nanoparticles: amplifying ROS stress to improve anticancer drug efficacy. Theranostics 2013;3:116-26. [PubMed]
- Klein S, Sommer A, Distel LV, et al. Superparamagnetic iron oxide nanoparticles as radiosensitizer via enhanced reactive oxygen species formation. Biochem Biophys Res Commun 2012;425:393-7. [PubMed]
- Huang C, Neoh KG, Xu L, et al. Polymeric nanoparticles with encapsulated superparamagnetic iron oxide and conjugated cisplatin for potential bladder cancer therapy. Biomacromolecules 2012;13:2513-20. [PubMed]
- Kettering M, Zorn H, Bremer-Streck S, et al. Characterization of iron oxide nanoparticles adsorbed with cisplatin for biomedical applications. Phys Med Biol 2009;54:5109-21. [PubMed]
- Raybaud-Diogène H, Fortin A, Morency R, et al. Markers of radioresistance in squamous cell carcinomas of the head and neck: a clinicopathologic and immunohistochemical study. J Clin Oncol 1997;15:1030-8. [PubMed]
- Shen LF, Chen J, Zeng S, et al. The superparamagnetic nanoparticles carrying the E1A gene enhance the radiosensitivity of human cervical carcinoma in nude mice. Mol Cancer Ther 2010;9:2123-30. [PubMed]
- Zhao D, Sun X, Tong J, et al. A novel multifunctional nanocomposite C225-conjugated Fe3O4/Ag enhances the sensitivity of nasopharyngeal carcinoma cells to radiotherapy. Acta Biochim Biophys Sin (Shanghai) 2012;44:678-84. [PubMed]
- Mallick S, Sun IC, Kim K, et al. Silica coated gold nanorods for imaging and photo-thermal therapy of cancer cells. J Nanosci Nanotechnol 2013;13:3223-9. [PubMed]
- Elbialy N, Mohamed N, Monem AS. Preparation and characterization of SiO2-Au nanoshells: in vivo study of its photo-heat conversion. J Biomed Nanotechnol 2013;9:158-66. [PubMed]
- Li J, Jiang H, Yu Z, et al. Multifunctional uniform core-shell Fe3O4@mSiO2 mesoporous nanoparticles for bimodal imaging and photothermal therapy. Chem Asian J 2013;8:385-91. [PubMed]
- Wang Y, Chen L, Liu P. Biocompatible triplex Ag@SiO2@mTiO2 core-shell nanoparticles for simultaneous fluorescence-SERS bimodal imaging and drug delivery. Chemistry 2012;18:5935-43. [PubMed]
- Zhao Z, Han Y, Lin C, et al. Multifunctional core-shell upconverting nanoparticles for imaging and photodynamic therapy of liver cancer cells. Chem Asian J 2012;7:830-7. [PubMed]
- Zhang Z, Wang L, Wang J, et al. Mesoporous silica-coated gold nanorods as a light-mediated multifunctional theranostic platform for cancer treatment. Adv Mater 2012;24:1418-23. [PubMed]
- Klein S, Dell’Arciprete ML, Wegmann M, et al. Oxidized silicon nanoparticles for radiosensitization of cancer and tissue cells. Biochem Biophys Res Commun 2013;434:217-22. [PubMed]
- Zhang Q, Yang W, Man N, et al. Autophagy-mediated chemosensitization in cancer cells by fullerene C60 nanocrystal. Autophagy 2009;5:1107-17. [PubMed]
- Sayes CM, Gobin AM, Ausman KD, et al. Nano-C60 cytotoxicity is due to lipid peroxidation. Biomaterials 2005;26:7587-95. [PubMed]
- Veeraraghavan J, Natarajan M, Lagisetty P, et al. Impact of curcumin, raspberry extract, and neem leaf extract on rel protein-regulated cell death/radiosensitization in pancreatic cancer cells. Pancreas 2011;40:1107-19. [PubMed]
- Tishler RB, Geard CR, Hall EJ, et al. Taxol sensitizes human astrocytoma cells to radiation. Cancer Res 1992;52:3495-7. [PubMed]
- Jin C, Bai L, Wu H, et al. Radiosensitization of paclitaxel, etanidazole and paclitaxel+etanidazole nanoparticles on hypoxic human tumor cells in vitro. Biomaterials 2007;28:3724-30. [PubMed]
- Werner ME, Cummings ND, Sethi M, et al. Preclinical evaluation of Genexol-PM, a nanoparticle formulation of paclitaxel, as a novel radiosensitizer for the treatment of non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;86:463-8. [PubMed]
- Xu WH, Han M, Dong Q, et al. Doxorubicin-mediated radiosensitivity in multicellular spheroids from a lung cancer cell line is enhanced by composite micelle encapsulation. Int J Nanomedicine 2012;7:2661-71. [PubMed]
- Werner ME, Copp JA, Karve S, et al. Folate-targeted polymeric nanoparticle formulation of docetaxel is an effective molecularly targeted radiosensitizer with efficacy dependent on the timing of radiotherapy. ACS Nano 2011;5:8990-8. [PubMed]
- Patt HM, Tyree EB, Straube RL, et al. Cysteine Protection against X Irradiation. Science 1949;110:213-4. [PubMed]
- Bari WA. Cysteine protection against the morphologic effects of x-irradiation on mouse spleen. Pathol Microbiol (Basel) 1968;32:205-18. [PubMed]
- Jagetia GC. Radioprotection and radiosensitization by curcumin. Adv Exp Med Biol 2007;595:301-20. [PubMed]
- Kumar KS, Srinivasan V, Toles R, et al. Nutritional approaches to radioprotection: vitamin E. Mil Med 2002;167:57-9. [PubMed]
- Murray D, Altschuler EM, Hunter N, et al. Protection by WR-3689 against gamma-ray-induced intestinal damage: comparative effect on clonogenic cell survival, mouse survival, and DNA damage. Radiat Res 1989;120:339-51. [PubMed]
- Capizzi RL, Oster W. Chemoprotective and radioprotective effects of amifostine: an update of clinical trials. Int J Hematol 2000;72:425-35. [PubMed]
- Pamujula S, Kishore V, Rider B, et al. Radioprotection in mice following oral delivery of amifostine nanoparticles. Int J Radiat Biol 2005;81:251-7. [PubMed]
- Theriot CA, Casey RC, Moore VC, et al. Dendro[C(60)]fullerene DF-1 provides radioprotection to radiosensitive mammalian cells. Radiat Environ Biophys 2010;49:437-45. [PubMed]
- Trajković S, Dobrić S, Jaćević V, et al. Tissue-protective effects of fullerenol C60(OH)24 and amifostine in irradiated rats. Colloids Surf B Biointerfaces 2007;58:39-43. [PubMed]
- Hwang M, Yong C, Moretti L, et al. Zebrafish as a model system to screen radiation modifiers. Curr Genomics 2007;8:360-9. [PubMed]
- Maurici D, Monti P, Campomenosi P, et al. Amifostine (WR2721) restores transcriptional activity of specific p53 mutant proteins in a yeast functional assay. Oncogene 2001;20:3533-40. [PubMed]
- Suresh Reddy J, Venkateswarlu V, et al. Radioprotective effect of transferrin targeted citicoline liposomes. J Drug Target 2006;14:13-9. [PubMed]
- Tarnuzzer RW, Colon J, Patil S, et al. Vacancy engineered ceria nanostructures for protection from radiation-induced cellular damage. Nano Lett 2005;5:2573-7. [PubMed]
- Vitolo JM, Cotrim AP, Sowers AL, et al. The stable nitroxide tempol facilitates salivary gland protection during head and neck irradiation in a mouse model. Clin Cancer Res 2004;10:1807-12. [PubMed]
- Bütof R, Dubrovska A, Baumann M. Clinical perspectives of cancer stem cell research in radiation oncology. Radiother Oncol 2013; [Epub ahead of print]. [PubMed]
- Peitzsch C, Kurth I, Kunz-Schughart L, et al. Discovery of the cancer stem cell related determinants of radioresistance. Radiother Oncol 2013; [Epub ahead of print]. [PubMed]
- Zaffaroni N, Pennati M, Daidone MG. Survivin as a target for new anticancer interventions. J Cell Mol Med 2005;9:360-72. [PubMed]
- Grdina DJ, Murley JS, Miller RC, et al. A survivin-associated adaptive response in radiation therapy. Cancer Res 2013;73:4418-28. [PubMed]
- Yamada T, Horinaka M, Shinnoh M, et al. A novel HDAC inhibitor OBP-801 and a PI3K inhibitor LY294002 synergistically induce apoptosis via the suppression of survivin and XIAP in renal cell carcinoma. Int J Oncol 2013;43:1080-6. [PubMed]
- Ling X, Cao S, Cheng Q, et al. A novel small molecule FL118 that selectively inhibits survivin, Mcl-1, XIAP and cIAP2 in a p53-independent manner, shows superior antitumor activity. PLoS One 2012;7:e45571 [PubMed]
- Pennati M, Millo E, Gandellini P, et al. RNA interference-mediated validation of survivin and Apollon/BRUCE as new therapeutic targets for cancer therapy. Curr Top Med Chem 2012;12:69-78. [PubMed]
- Zhang M, Mukherjee N, Bermudez RS, et al. Adenovirus-mediated inhibition of survivin expression sensitizes human prostate cancer cells to paclitaxel in vitro and in vivo. Prostate 2005;64:293-302. [PubMed]
- Gaca S, Reichert S, Rödel C, et al. Survivin-miRNA-loaded nanoparticles as auxiliary tools for radiation therapy: preparation, characterisation, drug release, cytotoxicity and therapeutic effect on colorectal cancer cells. J Microencapsul 2012;29:685-94. [PubMed]
- Jakus J, Yeudall WA. Growth inhibitory concentrations of EGF induce p21 (WAF1/Cip1) and alter cell cycle control in squamous carcinoma cells. Oncogene 1996;12:2369-76. [PubMed]
- Schmidt-Ullrich RK, Contessa JN, Lammering G, et al. ERBB receptor tyrosine kinases and cellular radiation responses. Oncogene 2003;22:5855-65. [PubMed]
- Rubin Grandis J, Melhem MF, Gooding WE, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst 1998;90:824-32. [PubMed]
- Patel JD. Epidermal growth factor receptor pathway targeted therapy in patients with aerodigestive malignancies. Curr Opin Oncol 2006;18:609-14. [PubMed]
- Ping Y, Jian Z, Yi Z, et al. Inhibition of the EGFR with nanoparticles encapsulating antisense oligonucleotides of the EGFR enhances radiosensitivity in SCCVII cells. Med Oncol 2010;27:715-21. [PubMed]
- Qiao Q, Jiang Y, Li G. Inhibition of the PI3K/AKT-NF-κB pathway with curcumin enhanced radiation-induced apoptosis in human Burkitt’s lymphoma. J Pharmacol Sci 2013;121:247-56. [PubMed]
- Elamin MH, Shinwari Z, Hendrayani SF, et al. Curcumin inhibits the Sonic Hedgehog signaling pathway and triggers apoptosis in medulloblastoma cells. Mol Carcinog 2010;49:302-14. [PubMed]