
Spot light on intestinal microbiota
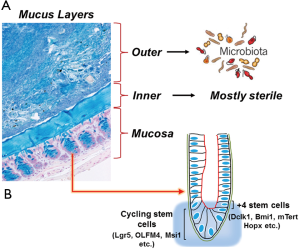
The human microbiome is diverse, varies between individuals and body sites, and is important in human health. The different compartments of the gastrointestinal tract are inhabited by populations of microorganisms. These living microorganisms form an enormous microbial community that includes both aerobic and anaerobic bacteria, as well as viruses, fungi and parasites (1). The human intestinal tract is populated by ~100 trillion bacteria, representing up to a thousand different genera and species (2). Bacterial numbers steadily increase from duodenum (102 bacteria/gram) to colon (~1012 bacteria/gram tissue) (3). The mucus layer generates additional heterogeneity by separating the bacteria confined to the intestinal lumen from those able to invade below the mucus and attach to the epithelium (Figure 1) (4). Mucus in the gastrointestinal tract adheres as a gel to surface epithelium producing an interface between the lumen and the mucosa. In mice, colonic mucus secreted by goblet cells, consists of two layers extending 150 m above the epithelial cells (Figure 1A). The outer layer is movable, has an expanded volume due to proteolysis of MUC2 mucin, and is colonized by bacteria (Figure 1A) (5). The inner layer is densely packed, firmly attached to the epithelium, and usually devoid of bacteria. Proteases of certain parasites and some bacteria can cleave mucins and dissolve the mucus as part of their pathogenicity. The inner mucus layer can also become penetrable to bacteria by several other mechanisms, including aberrations in the immune system. When bacteria reach the epithelial surface, the immune system is activated and inflammation is triggered. During inflammatory bowel disease (IBD) for example, contact between intestinal bacteria and the mucosal surfaces may trigger and perpetuate the colonic inflammation (6).
The bacterial communities vary in composition along the digestive tract with each individual harboring a unique collection of species and adapt through life according to lifestyle and nutrition of the host. In both human and the murine intestine, the dominant microbiota belongs to the Firmicutes and Bacteroidetes phyla. In addition, Proteobacteria, Actinobacteria, Verrucomicrobia, Cyanobacteria and Deferribacteres have also been detected in humans and mice, and Fusobacteria in humans (7). The intestinal microbiota contributes to bowel health in the host by fermenting unused energy substrates (8,9), preventing growth of harmful pathogenic bacteria (10), and assisting the host immune system (11-13). On the other hand, disordered and impaired microbiota communities are associated with conditions such as obesity (14), IBD (15,16), and cancerous lesions of the intestine, liver and pancreas (Table 1) (17). The abundances of tissue- or mucosa-associated microbiota members in IBD (Crohn’s disease and ulcerative colitis), and colorectal cancer (CRC) patients are altered as compared to healthy controls (18,19). Interestingly, population sizes for members of the Enterobacteriaceae family (e.g., Escherichia coli, Klebsiella, and Shigella), which are commonly associated with inflammation, increase in all three diseases. Sobhani et al. (19) reported higher levels of Bacteroides and Prevotella found in the stool of patients with CRC than in the stool of patients with normal colonoscopy. Two recent studies found Fusobacterium associated with CRC tissue but not normal colon (20,21). It is therefore imperative to investigate therapeutic strategies aimed at manipulating the dysbiosis (disordered microbiobial community) influenced by stressors (disease or other factors). This could assist the endogenous microbiota in restoring a normal or more consolidated microbiota status. Ingested probiotics (health-promoting bacteria) or their released factors could alter the endogenous microbiota to achieve a beneficial balance in the bowel. Indeed, well-designed, randomized and double blind placebo-controlled human studies using pro- pre- or synbiotics, with adequate follow-up are mandated to formulate directions for prevention and therapy. Together the combined microbiota could reduce the severity of certain diseases while preventing others, ultimately improving human health.

Full table
Interventions that affect the microbiota
Antibiotics
Clinical use of antibiotics, while facilitating clearance of targeted infections, also disrupts commensal microbial communities and decreases host resistance to antibiotic-resistant microbes. Antibiotics have a radical effect on the GI microbial community at all stages of life, and responses vary among individuals. Global use of antibiotics and disappearing indigenous lifestyles could be eliminating a key source of information about the microbes with which we have evolved; preserving samples of these endangered GI tract microbes, at all stages of human development, may be an important goal of the expanded human microbiome projects worldwide. The mammalian intestine harbors a complex microbial community that provides numerous benefits to its host. However, the microbiota can also include potentially virulent species, termed pathobiont, which can cause disease when intestinal homeostasis is perturbed. The molecular mechanisms by which pathobionts cause disease remain poorly understood. A study recently described a sepsis-like disease that occurs upon gut injury in antibiotic-treated mice (22). Sepsis was associated with the systemic spread of a specific multidrug-resistant Escherichia coli pathobiont that expanded markedly in the microbiota of antibiotic-treated mice. Rapid sepsis-like death required a component of the innate immune system, the Naip5-Nlrc4 inflammasome. In accordance with Koch’s postulates, it was discovered that E. coli pathobiont was sufficient to activate Naip5-Nlrc4 and cause disease when injected intravenously into unmanipulated mice. These findings reveal how sepsis-like disease can result from recognition of pathobionts by the innate immune system.
Radiation
Exposure to high doses of ionizing radiation (IR) in humans causes hair loss, skin burns, birth defects, gastrointestinal illness, cancer and death. Cells that undergo rapid cell division and exhibit rapid proliferation are the most sensitive to the cytotoxic and genotoxic effects of radiation injury. These tissues include the bone marrow, reproductive and lymphoid tissue and the gastrointestinal tract (23). The gastrointestinal response to acute radiation injury is the most extensively characterized model system for studying injury-repair in the rodent. The continuous and rapid turnover of the gastrointestinal tract in particular explains its unique sensitivity to high doses of IR. Following severe DNA damage, the cell has two physiological options, (I) repair the DNA damage or (II) die. Following high dose radiation (>8 Gy; radiation response is species and dose-dependent) gastrointestinal stem/progenitor epithelial cells are unable to sufficiently multiply to replace dying cells. This results in malabsorption syndromes, electrolyte imbalances, fluid loss and bacterial infections. Agents that can prevent or attenuate epithelial stem/progenitor cell loss and thereby prevent barrier disruption or restore epithelial integrity would be ideal candidates for radioprotective drug development.
Radiation influences and disturbs the mucosal microbiota, leading to a translocation of microorganisms or microbial products through the mucosa into the blood circulation (24). Mucosal permeability of irradiated colon of patients treated for rectal cancer can be expected to be increased due to the mucosal atrophy observed in the irradiated patients and may result in an increased risk of radiation enteritis (25). Translocation of pathogenic organisms through the intestinal wall into the bloodstream, the peritoneal cavity and abdominal organs is a well-recognized cause of supervening sepsis and life-threatening complications in critically ill patients (26). There is an urgent need therefore for rapid, accurate, and sensitive diagnostic platforms to confirm exposure to radiation and estimate the dose absorbed by individuals subjected to acts of radiological terrorism, nuclear power plant accidents, or nuclear warfare. Clinical symptoms and physical dosimeters, even when available, do not provide adequate diagnostic information to triage and treat life-threatening radiation injuries. In a recent study, Lam V. et al. (27) elegantly described presence of 12 members of Bacteroidales, Lactobacillaceae, and Streptococcaceae in human and rat feces after radiation exposure while levels of 98 Clostridiaceae and Peptostreptococcaceae family members and decreased levels of 47 separate Clostridiaceae members were recorded. Thus, intestinal microbiota serves as novel biomarkers of prior radiation exposure, and may be able to complement conventional chromosome aberrational analysis to significantly enhance biological dose assessments (27). Indeed, investigations aimed at deciphering the microbiome-host interactions before and after small bowl radiation injury may eventually allow prediction of disease course and offer opportunities for the development of novel therapeutic or prophylactic strategies. Finally, the microbiota composition can be monitored to determine the beneficial effects of the targeted approach during radio-sensitization using gold and other nanoparticles. Such studies could prove invaluable towards improving the efficacy of targeted approach and also help in assessing whether they are indeed limiting/reducing toxicity of radiation therapy.
Chemotherapy
Systemic cytotoxic chemotherapy is a common treatment for malignancies which has been in use for half a century (28). It can cause functional and structural changes to the gastrointestinal tract (29) altering the host environment. Common gastrointestinal symptoms following chemotherapy include heartburn, abdominal pain, diarrhea (and constipation), bloating and nausea (29). These symptoms arise as the result of the damage caused by chemotherapy agents (29). Abdominal pain is caused by the extensive damage occurring in the small intestine. Diarrhea and constipation are thought to be caused by the alteration in absorptive functions of cells, goblet cell and mucin distribution and composition, and bacterial interactions with these cells and metabolites of the drugs themselves (29). Several studies have demonstrated the effects of chemotherapy agents on the intestinal microbiota (30). One of the most investigated agents is irinotecan, due to the involvement of intestinal microbiota in its metabolism (31,32). Irinotecan (used to treat a variety of solid tumors) is a topoisomerase-I inhibitor, converted to active and toxic metabolite SN-38, before being further metabolized to non-toxic metabolite SN-38 glucuronide (SN-38G) (31,32). This molecule is excreted into the gastrointestinal tract where it becomes susceptible to processing by bacterial enzymes. Intestinal microbes produce the enzyme β-glucuronidase, which can cleave the glucuronide molecule from the less toxic metabolite of irinotecan, rendering it re-activated and toxic (31,32). The effects on the intestinal microbiota caused by irinotecan have been considered important due to this involvement in the drug’s metabolism and potentially compounded toxicities. Antimetabolite 5-Fluorouracil (5-FU), used to treat colorectal, breast and liver cancers, has been shown to be associated with changes to the intestinal microbiota (33). In clinical studies, changes to intestinal microbiota have also been demonstrated. In a study by van Vliet and colleagues in 2009 (34), paediatric acute myeloid leukaemia patients receiving AML-97 were shown to have decreased intestinal microbial diversity using denaturing gradient gel electrophoresis. Cancer patients receiving chemotherapy often develop mucositis as a direct result of their treatment. Recently, the intestinal microbiota has attracted significant attention in the investigation of the pathobiology of mucositis. Moreover, chemotherapy-induced diarrhea (CID) is a common problem, especially in patients with advanced cancer. The incidence of CID has been reported to be as high as 50-80% of treated patients especially with 5-FU bolus or some combination therapies of irinotecan and fluoropyrimidines. Probiotics have been shown to prevent diarrhea in inflammatory bowel disease. Preclinical data yielded a similar efficacy in CID (35,36). In the clinical setting, a combination of Lactobacillus rhamnosus and fiber resulted in a significant reduction of grade 3/4 diarrhea in a randomized study of patients treated with either bolus (Mayo) or bolus and infusional (simplified de Gramont) 5-FU with leucovorin for adjuvant treatment of CRC (37). In a recent Interscience Conference on Antimicrobial Agents and Chemotherapy, data presented suggest that changes in intestinal microbiota, especially increases in populations of Bacteroides, predict which chemotherapy patients are likely to develop CID. These changes occur a median of 6 days before the onset of symptoms, which could pave the way for probiotic or other targeted therapies. With significant effects on the intestinal microbiota occurring following the administration of chemotherapy, there is now interest surrounding the downstream pathological effects that may be associated with the altered intestinal ecology and use of nanotechnology to deliver chemotherapeutic drugs to reduce such toxicities (ref).
Diet and the microbiome: role of pro, pre and synbiotics
Diet and environmental exposures influence the composition of the gut microflora. The selected bacterial populations might, in turn, affect the physiologic performance of the human host. Three enterotypes have been identified to classify people by their predominant classes of intestinal microbiota (7). These bacterial profiles are stable, even with short-term diet changes (38,39). As these studies and others have shown, the composition of the intestinal flora varies little over time (7,40), but the relative distribution of bacterial types within the population can be affected by diet, medication, and behaviors such as smoking (41,42). Bacteria such as Bifidobacter species and Lactobacilli, popularly known as probiotics are defined as viable microorganisms, sufficient amounts of which reaches the intestine in an active state and thus exerts positive health effects (43). Popular probiotic species used commercially include L. paracasei, L. rhamnosus, L. acidophilus, L. johnsonii, L. fermentum, L. reuteri, L. plantarum, Bifidobacterium longum and Bifidobacterium animalis. Among the numerous health benefits attributed to probiotics, the (transient) modulation of the intestinal microflora of the host and the capacity to interact with the immune system directly or mediated by the autochthonous microflora, are basic mechanisms. In addition, well-established probiotic effects include but not limited to: prevention and/or reduction of duration and complaints of rotavirus-induced or antibiotic-associated diarrhea, reduction of the levels of cancer-promoting enzymes and/or metabolites in the gut, beneficial effects associated with restoration of commensals, normalization of bowel movement and stool consistency in subjects suffering from constipation or an irritable colon, prevention or alleviation of allergies and atopic diseases in infants and finally, prevention of respiratory tract infections and other infectious diseases.
A prebiotic is “a selectively fermented ingredient that allows specific changes, both in the composition and/or activity in the gastrointestinal microflora that confers benefits upon host’s well-being and health” (44), whereas synergistic combinations of pro- and prebiotics are called synbiotics. Prebiotics are dietary fibers with a well-established positive impact on the intestinal microflora. Other health effects of prebiotics (prevention of diarrhea or obstipation, modulation of the metabolism of the intestinal flora, cancer prevention, positive effects on lipid metabolism, stimulation of mineral adsorption and immunomodulatory properties) are indirect, i.e. mediated by the intestinal microflora. Pectin for example, is a polysaccharide fiber, which has a broad anti-inflammatory properties and undergoes fermentation in the colon to generate short chain fatty acids (SCFAs) (45), in particular, acetate, n-butyrate, propionate, and valerate. These SCFAs are utilized by epithelial cells in the gut and are excreted in stool. Butyrate has anti-proliferative properties in vitro and anti-cancerous properties in mouse models (46). Correlations between butyrate concentrations and CRC incidence in humans have been difficult to assess due in part to existence of CRC subtypes, levels and timing of butyrate administration. Butyrate supplementation if given orally is rapidly absorbed in the upper GI tract and does not reach the colon, but a recent study showed that oral administration of Butyrivibrio fibrisolvens, a butyrate-producing bacterium, decreases the formation of aberrant crypt foci in the colon and rectum of mice (47). We have shown in an in vivo model of Citrobacter rodentium (CR)-induced colonic crypt hyperproliferation and hyperplasia that dietary pectin as a source of butyrate is an effective means to deliver butyrate to the colon (48-50). In an ongoing study, we have discovered that intervention through dietary pectin (6%) promotes recruitment of commensals such as B. vulgatus, S. gordonii and L. lactis that are lost during Citrobacter infection-induced colitis (manuscript in preparation). Moreover, in susceptible hosts, when CR-infected mice were placed on 6% pectin diet for 9 days starting 2 days post-CR infection, it: (I) reduced the morbidity and mortality of CR-infected C3H mice; (II) Restored the junctional labeling of β-catenin/E-cadherin and ZO-2 and; (III) Restored the barrier function. Additionally, we have also found that mice treated with dietary pectin and subjected to 14 Gy irradiation (IR) had significant crypt stem cell survival following microcolony assay (unpublished observation). Furthermore, mice treated with pectin had improvement in overall survival compared to mice treated with normal diet. Thus, pectin administration following severe IR can mitigate radiation-induced deletion of gut stem and/or progenitor cells, facilitate crypt regeneration, enhance mucosal barrier function and ultimately promote survival thereby justifying the use of prebiotics. Indeed, as mentioned elsewhere, use of pectin is found associated with restoration of commensals that promote survival and better outcome.
Intestinal stem cells (ISCs) and the microbiota
ISCs are undifferentiated primitive cells that divide asymmetrically, undergo self-renewal and produce committed daughter cells that contribute towards all adult lineages within the intestine. The location and identity of ISCs has been a subject of much debate, with implications for understanding gastrointestinal cancer, repair after intestinal injury, and normal physiology. Numerous reports have suggested that +4 cells correspond to the location of slow-cycling, label-retaining cells (51) that stain positive for Bmi1, mTert and DCLK1 (52-54) whereas a different stem cell niche located at the crypt base and occupied by crypt base columnar (CBC) cells are marked by Lgr5 (55) (Figure 1B). Although +4 cells and CBCs are clearly distinct, lineage-tracing studies have shown that both can give rise to all the lineages in the intestine: goblet cells, entero-endocrine cells, Paneth cells, and epithelial absorptive cells. An elegant study recently showed that Hopx, an atypical homeobox protein, is a specific marker of +4 cells (56) (Figure 1B). Hopx-expressing cells gave rise to CBCs and all mature intestinal epithelial lineages. Conversely, CBCs were found to give rise to +4 Hopx-positive cells. While these findings demonstrate a bidirectional lineage relationship between active and quiescent stem cells in their niches, a recent study suggested that +4 cells expressing Bmi1 can compensate for the loss of CBCs to maintain homeostasis after experimental ablation of Lgr5-expressing cells (57). It is therefore likely that the Lgr5 population is an ‘‘active’’ stem cell pool, which can be replenished from a more quiescent Bmi1 pool of cells. These findings have further provided us tools to visualize stem cells and examine their behavior in the context of self-renewal and multi-potency, two characteristics of the adult stem cells. Self-renewal is the process that requires stringent cell cycle control and often maintenance of multi-potency or pluripotency, depending on the stem cell. The homeostatic activity of the adult stem cell reservoirs is tailored to meet the specific renewal requirements of individual tissues through a combination of intrinsic genetic programming and local cues delivered from the surrounding environment (the niche). In the intestine, various signaling pathways including Wnt, Notch, and BMP act in concert on the crypt base to maintain stem cell-driven epithelial renewal (58). In response to changing tissue demands, stem cells undergo changes in cell cycle status and developmental potential over time, requiring different self-renewal programs at different stages of life. Reduced stem cell function and tissue regenerative capacity during aging are caused by changes in self-renewal programs that augment tumor suppression. Cancer arises from mutations that inappropriately activate self-renewal programs.
Somatic stem cells of the colon sustain self-renewal and perturbations in stem cell dynamics are generally believed to represent the earliest step towards colon carcinogenesis. Many tumors consist of phenotypically and functionally heterogeneous cancer cells. For many years, such heterogeneity was based on the notion that all cancer cells possess tumorigenic potential and can develop tumor dependent on genetic and/or epigenetic changes. Recent studies however, have suggested that tumors show hierarchy wherein, a subpopulation of cancer cells may have a higher tumorigenic potential than other cancer cells. These cells, popularly known as ‘cancer stem cells’ (CSCs), are defined as ‘‘a cell within a tumor that possesses the capacity to self-renew and to cause the heterogeneous lineages of cancer cells that comprise the tumor.’’ By crossing stem-cell-specific Lgr5-EGFP-IRES-creERT2 knock-in mice to Apcflox/flox mice, it was unequivocally shown that crypt Lgr5+ stem cells are the cells-of-origin of intestinal cancer (59). A recent study further strengthened this claim by showing that Doublecortin-like kinase 1 (Dclk1) labeled tumor stem cells (TSCs) continuously produced tumor progeny in the polyps of ApcMin/+ mice (60). Specific ablation of Dclk1-positive TSCs resulted in a marked regression of polyps without apparent damage to the normal intestine (60).
The intestinal crypt harboring the stem cells is the site of epithelial restitution and represents a rare situation in which a differentiating and proliferative epithelium is directly exposed to bacteria, both permanent symbionts and occasional pathogens. It is therefore likely that coevolution of mammals with their gut microbiota has led to a balance, protecting the crypt against microbial insults while maintaining a capacity to sense and integrate microbial signals to convert them into signals boosting epithelial regeneration (61). Metagenomic studies involving whole-gut luminal microbiome recently revealed great opportunities for physiological and pathophysiological analysis of the host-microbiota interface. On this basis, it appears increasingly important to analyze which niches of the gut exposed to a particular microbiota are of major functional importance. A unique epithelial subset, called tuft cells, resides in the gastrointestinal tract of many vertebrates and recent studies have suggested that apical "tuft" may detect and transmit environmental signals. It is therefore likely that the intestinal tuft cells sense gut microbes via taste receptors and contribute to gut homeostasis. Interestingly, we and others have shown that Dclk1 marks a quiescent stem cell population in the gut (54,62). Since Dclk1 has also been shown to label tuft cells (63), a provocative hypothesis can be envisaged wherein, stem cells may be directly implicated in sensing and responding to gut microbiota. Likewise, interventions such as antibiotic treatment, radiation or chemotherapy that affect the microbiota are expected to target ISCs to modulate disease pathogenesis. However, no such link between the environmental cues and the ISCs has been established so far. A plausible approach dealing with utilization of biocompatible nanoparticles encapsulated with phytonutrients to restore favorable microbiome may prove strategically useful for preventing or minimizing deregulated stem cells. Indeed, an elegant study recently showed that Lgr5+ stem cells are highly susceptible to DSS-induced damage and that dietary cues can impact stem cells’ regulatory networks (64). Similarly, increases in Lgr5+ stem cell number in mice exposed to calorie restriction have been described (65). This illustrates the point that we do not yet know much about how diet and microbiota overall affect stem cell number and function, interconversion of cells among different stem cell compartments, or the intestinal “niche”. Given the availability of Lgr5, Bmi1 and Prominin-1 reporter mice, it will be fascinating to perform lineage tracing wherein, direct stool/microbial implantation into these mice raised under germ-free conditions will allow establishing a direct role for microbiota on stem cells which can be objectively quantified. Similarly, targeted approach in monitoring the landscape and the status of the stem cells using fluorescent nanoparticles (in animal studies) or MRI contrast nanoparticles (future clinical studies) may be helpful in tracking stem cells within the gastrointestinal tract. Finally, biocompatible nanoparticles encapsulated with phytonutrients to restore favorable microbiome could prove invaluable in protecting stem cells following radiation therapy.
Nanoparticles and other carriers for pro/prebiotics to improve human health
The organic construct consumed as food comes packaged in units that carry the active components and protect the entrapped active materials until delivered to targeted human organs. The packaging and delivery role is mimicked in the microencapsulation tools used to deliver active ingredients in processed foods. Microencapsulation efficiency is balanced against the need to access the entrapped nutrients in bioavailable forms. Encapsulated ingredients boosted with bioactive nutrients are intended for improved health and well-being and to prevent future health problems. Presently, active ingredients are delivered using new techniques, such as hydrogels, nanoemulsions, and nanoparticles. Utilizing the nanoparticle encapsulated siRNA approach to block Wnt/-catenin signaling, we have recently shown that we can significantly attenuate the process of epithelial-mesenchymal transition (EMT) induced by bacterial infection (66). Similar approaches to encapsulate pro- or pre-biotics will have to be adapted to improve delivery in order to target diseases with infectious etiology. In the future, nutraceuticals and functional foods may be tailored to individual metabolic needs and tied to each person’s genetic makeup. Bioactive ingredients provide health-enhancing nutrients and are protected through encapsulation processes that shield the active ingredients from deleterious environments.
The colon provides a plethora of therapeutic opportunities. There are multiple disease targets, drug molecules, and colon-specific delivery systems to be explored. Clinical studies highlight the potential for systemic delivery via the colon, and the emerging data on the levels of cell membrane transporters and metabolic enzymes along the gut could prove advantageous for this. Often efflux transporters and metabolic enzyme levels are lower in the colon, suggesting a potential for improved bioavailability of drug substrates at this site. Local delivery to the colonic mucosa remains a valuable therapeutic option. New therapies that target inflammatory mediators could improve the treatment of IBD, and old and new anticancer molecules could, when delivered topically, prove to be beneficial adjuncts to the current systemic or surgical treatments. New issues such as pharmacogenomics, chronotherapeutics, and the delivery of prebiotics and probiotics are some of the recent advances to improve human health. Targeting drugs to the colon utilizes various strategies, each with their advantages and flaws. The most promising systems are considered in the light of the physiological data which influence their in vivo behavior. Biocompatible nanoparticles carrying payloads (nutrients/drugs) can be monitored during gastrointestinal transit (Figure 2A) and potentially be targeted to specific gastrointestinal compartments (Figure 2B).
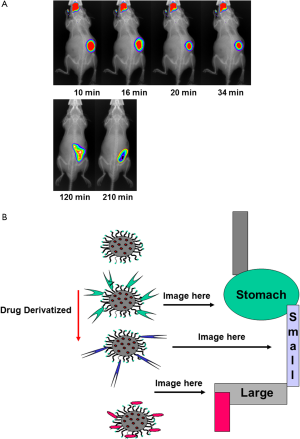
Microencapsulation has been developed by the pharmaceutical industry as a means to control or modify the release of drug substances from drug delivery systems. In drug delivery systems microencapsulation is used to improve the bioavailability of drugs, control drug release kinetics, minimize drug side effects, and mask the bitter taste of drug substances. The application of microencapsulation has been extended to the food industry, typically for controlling the release of flavorings and the production of foods containing functional ingredients (e.g., probiotics and bioactive ingredients). The type of microcapsule produced and its resultant release properties are dependent on the microencapsulation technology, in addition to the physicochemical properties of the core and the shell materials. The key criteria in selecting a suitable microencapsulation technology are also discussed. Two of the most common physical microencapsulation technologies used in pharmaceutical processing, fluidized-bed coating, and extrusion-spheronization might be adapted to the microencapsulation of functional bioactive ingredients in the food industry.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Nanotechnology in Radiation Research”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.08.12). The series “Nanotechnology in Radiation Research” was commissioned by the editorial office without any funding or sponsorship. RVLP served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Translational Cancer Research. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moens E, Veldhoen M. Epithelial barrier biology: good fences make good neighbours. Immunology 2012;135:1-8. [PubMed]
- Clemente JC, Ursell LK, Parfrey LW, et al. The impact of the gut microbiota on human health: an integrative view. Cell 2012;148:1258-70. [PubMed]
- Frank DN, Pace NR. Molecular-phylogenetic analyses of human gastrointestinal microbiota. Curr Opin Gastroenterol 2001;17:52-7. [PubMed]
- Pullan RD, Thomas GA, Rhodes M, et al. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut 1994;35:353-9. [PubMed]
- Johansson ME, Phillipson M, Petersson J, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A 2008;105:15064-9. [PubMed]
- Podolsky DK. The current future understanding of inflammatory bowel disease. Best Pract Res Clin Gastroenterol 2002;16:933-43. [PubMed]
- Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature 2011;473:174-80. [PubMed]
- Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027-31. [PubMed]
- Flint HJ, Bayer EA, Rincon MT, et al. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol 2008;6:121-31. [PubMed]
- Dai ZL, Wu G, Zhu WY. Amino acid metabolism in intestinal bacteria: links between gut ecology and host health. Front Biosci (Landmark Ed) 2011;16:1768-86. [PubMed]
- Corr SC, Hill C, Gahan CG. Understanding the mechanisms by which probiotics inhibit gastrointestinal pathogens. Adv Food Nutr Res 2009;56:1-15. [PubMed]
- Kosiewicz MM, Zirnheld AL, Alard P. Gut microbiota, immunity, and disease: a complex relationship. Front Microbiol 2011;2:180. [PubMed]
- DuPont AW, DuPont HL. The intestinal microbiota and chronic disorders of the gut. Nat Rev Gastroenterol Hepatol 2011;8:523-31. [PubMed]
- Ley RE, Bäckhed F, Turnbaugh P, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 2005;102:11070-5. [PubMed]
- Mueller C, Macpherson AJ. Layers of mutualism with commensal bacteria protect us from intestinal inflammation. Gut 2006;55:276-84. [PubMed]
- Dicksved J, Halfvarson J, Rosenquist M, et al. Molecular analysis of the gut microbiota of identical twins with Crohn’s disease. ISME J 2008;2:716-27. [PubMed]
- Spor A, Koren O, Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol 2011;9:279-90. [PubMed]
- Marchesi JR, Dutilh BE, Hall N, et al. Towards the human colorectal cancer microbiome. PLoS One 2011;6:e20447 [PubMed]
- Sobhani I, Tap J, Roudot-Thoraval F, et al. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS One 2011;6:e16393 [PubMed]
- Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 2012;22:299-306. [PubMed]
- Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 2012;22:292-8. [PubMed]
- Ayres JS, Trinidad NJ, Vance RE. Lethal inflammasome activation by a multidrug-resistant pathobiont upon antibiotic disruption of the microbiota. Nat Med 2012;18:799-806. [PubMed]
- Wakeford R. Radiation effects: Modulating factors and risk assessment–an overview. Ann ICRP 2012;41:98-107. [PubMed]
- Packey CD, Ciorba MA. Microbial influences on the small intestinal response to radiation injury. Curr Opin Gastroenterol 2010;26:88-94. [PubMed]
- Zimmerer T, Böcker U, Wenz F, et al. Medical prevention and treatment of acute and chronic radiation induced enteritis--is there any proven therapy? A short review. Z Gastroenterol 2008;46:441-8. [PubMed]
- Brook I, Elliott TB, Ledney GD, et al. Management of postirradiation sepsis. Mil Med 2002;167:105-6. [PubMed]
- Lam V, Moulder JE, Salzman NH, et al. Intestinal microbiota as novel biomarkers of prior radiation exposure. Radiat Res 2012;177:573-83. [PubMed]
- Crawford S. Is it time for a new paradigm for systemic cancer treatment? Lessons from a century of cancer chemotherapy. Front Pharmacol 2013;4:68. [PubMed]
- Stringer AM, Gibson RJ, Bowen JM, et al. Chemotherapy-induced mucositis: the role of gastrointestinal microflora and mucins in the luminal environment. J Support Oncol 2007;5:259-67. [PubMed]
- van Vliet MJ, Harmsen HJ, de Bont ES, et al. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog 2010;6:e1000879 [PubMed]
- Roberts AB, Wallace BD, Venkatesh MK, et al. Molecular insights into microbial β-glucuronidase inhibition to abrogate CPT-11 toxicity. Mol Pharmacol 2013;84:208-17. [PubMed]
- Lin XB, Dieleman LA, Ketabi A, et al. Irinotecan (CPT-11) chemotherapy alters intestinal microbiota in tumour bearing rats. PLoS One 2012;7:e39764 [PubMed]
- Holma R, Korpela R, Sairanen U, et al. Colonic methane production modifies gastrointestinal toxicity associated with adjuvant 5-fluorouracil chemotherapy for colorectal cancer. J Clin Gastroenterol 2013;47:45-51. [PubMed]
- van Vliet MJ, Tissing WJ, Dun CA, et al. Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin Infect Dis 2009;49:262-70. [PubMed]
- Von Bültzingslöwen I, Adlerberth I, Wold AE, et al. Oral and intestinal microflora in 5-fluorouracil treated rats, translocation to cervical and mesenteric lymph nodes and effects of probiotic bacteria. Oral Microbiol Immunol 2003;18:278-84. [PubMed]
- Gibson RJ, Bowen JM, Alvarez E, et al. Establishment of a single-dose irinotecan model of gastrointestinal mucositis. Chemotherapy 2007;53:360-9. [PubMed]
- Osterlund P, Ruotsalainen T, Korpela R, et al. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. Br J Cancer 2007;97:1028-34. [PubMed]
- Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334:105-8. [PubMed]
- Gophna U. Microbiology. The guts of dietary habits. Science 2011;334:45-6. [PubMed]
- Zoetendal EG, Akkermans AD, De Vos WM. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl Environ Microbiol 1998;64:3854-9. [PubMed]
- Jernberg C, Löfmark S, Edlund C, et al. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 2010;156:3216-23. [PubMed]
- Parry SD, Barton JR, Welfare MR. Factors associated with the development of post-infectious functional gastrointestinal diseases: does smoking play a role? Eur J Gastroenterol Hepatol 2005;17:1071-5. [PubMed]
- Ciorba MA, Stenson WF. Probiotic therapy in radiation-induced intestinal injury and repair. Ann N Y Acad Sci 2009;1165:190-4. [PubMed]
- Delzenne NM, Neyrinck AM, Cani PD. Modulation of the gut microbiota by nutrients with prebiotic properties: consequences for host health in the context of obesity and metabolic syndrome. Microb Cell Fact 2011;10:S10. [PubMed]
- O’Keefe SJ. Nutrition and colonic health: the critical role of the microbiota. Curr Opin Gastroenterol 2008;24:51-8. [PubMed]
- Roy CC, Kien CL, Bouthillier L, et al. Short-chain fatty acids: ready for prime time? Nutr Clin Pract 2006;21:351-66. [PubMed]
- Ohkawara S, Furuya H, Nagashima K, et al. Oral administration of butyrivibrio fibrisolvens, a butyrate-producing bacterium, decreases the formation of aberrant crypt foci in the colon and rectum of mice. J Nutr 2005;135:2878-83. [PubMed]
- Umar S, Morris AP, Kourouma F, et al. Dietary pectin and calcium inhibit colonic proliferation in vivo by differing mechanisms. Cell Prolif 2003;36:361-75. [PubMed]
- Chandrakesan P, Ahmed I, Anwar T, et al. Novel changes in NF-{kappa}B activity during progression and regression phases of hyperplasia: role of MEK, ERK, and p38. J Biol Chem 2010;285:33485-98. [PubMed]
- Chandrakesan P, Ahmed I, Chinthalapally A, et al. Distinct compartmentalization of NF-κB activity in crypt and crypt-denuded lamina propria precedes and accompanies hyperplasia and/or colitis following bacterial infection. Infect Immun 2012;80:753-67. [PubMed]
- Potten CS, Kovacs L, Hamilton E. Continuous labelling studies on mouse skin and intestine. Cell Tissue Kinet 1974;7:271-83. [PubMed]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 2008;40:915-20. [PubMed]
- Montgomery RK, Carlone DL, Richmond CA, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A 2011;108:179-84. [PubMed]
- May R, Riehl TE, Hunt C, et al. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells 2008;26:630-7. [PubMed]
- Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007;449:1003-7. [PubMed]
- Takeda N, Jain R, LeBoeuf MR, et al. Interconversion between intestinal stem cell populations in distinct niches. Science 2011;334:1420-4. [PubMed]
- Tian H, Biehs B, Warming S, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 2011;478:255-9. [PubMed]
- Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol 2009;174:715-21. [PubMed]
- Barker N, Ridgway RA, van Es JH, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009;457:608-11. [PubMed]
- Nakanishi Y, Seno H, Fukuoka A, et al. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet 2013;45:98-103. [PubMed]
- Moossavi S, Zhang H, Sun J, et al. Host-microbiota interaction and intestinal stem cells in chronic inflammation and colorectal cancer. Expert Rev Clin Immunol 2013;9:409-22. [PubMed]
- May R, Sureban SM, Hoang N, et al. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells 2009;27:2571-9. [PubMed]
- Saqui-Salces M, Keeley TM, Grosse AS, et al. Gastric tuft cells express DCLK1 and are expanded in hyperplasia. Histochem Cell Biol 2011;136:191-204. [PubMed]
- Davidson LA, Goldsby JS, Callaway ES, et al. Alteration of colonic stem cell gene signatures during the regenerative response to injury. Biochim Biophys Acta 2012;1822:1600-7.
- Yilmaz ÖH, Katajisto P, Lamming DW, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 2012;486:490-5. [PubMed]
- Chandrakesan P, Roy B, Jakkula LU, et al. Utility of a bacterial infection model to study epithelial-mesenchymal transition, mesenchymal-epithelial transition or tumorigenesis. Oncogene 2013; [Epub ahead of print]. [PubMed]


