
Differences between human and rodent DNA-damage response in hematopoietic stem cells: at the crossroads of self-renewal, aging and leukemogenesis
“To be, or not to be, that is the question…”
William Shakespeare, Hamlet, ca. 1604
Introduction
Hematopoiesis is a highly regulated process that supplies mature blood cells of various lineages. In an adult human, around 1011 new blood cells are produced daily to ensure homeostasis. To meet this high regenerative demand, hematopoiesis is structured as a cellular hierarchy composed of cells endowed with different proliferative, differentiation and longevity potentials (1,2). Hematopoietic stem cells (HSCs) are located at the apex of the hierarchy and maintain blood production due to their unique ability to produce more blood stem cells (a property defined as self-renewal), as well as to give rise to multipotent progenitors with limited self-renewal capacity (2,3). Short-lived but extremely proliferative lineage-committed progenitors (CPs), which are the progeny of multipotent progenitors, generate large numbers of differentiated cells to ensure daily homeostasis (4,5). During injury or infection, stem and progenitor compartments undergo expansion via replication to meet the increased demand for particular cell subsets, followed by a return to homeostasis.
All cells in the body, including highly proliferative and long-lived hematopoietic subsets, must constantly contend with different types of DNA damage. Most of this damage is generated by endogenous sources, such as reactive oxygen species (ROS). In addition, exogenous DNA insults, such as chemotherapeutic drugs and ionizing radiation (IR), can induce single- and double-strand breaks (DSBs). It has been estimated that the average cell experiences approximately 100,000 spontaneous DNA lesions daily (6). To protect genome integrity, organisms have developed highly sophisticated DNA-damage response (DDR) pathways. The critical importance of the DDR in hematopoiesis is well demonstrated by the severe clinical consequences-including bone marrow (BM) failure, immunodeficiency and a high incidence of hematological cancers-observed in patients with inherited mutations in DNA damage signaling and repair components [e.g., Ataxia telangiectasia mutated (ATM) and Fanconi anemia (FA) genes respectively] (7). Analysis of human HSC DNA isolated from newborn, young and elderly individuals by whole-genome sequencing has shown that long-lived self-renewing HSC serve as a reservoir for DNA-damage accumulation and thus represent a likely cell of origin for hematopoietic malignancies (8,9). Obviously, the short life span of hematopoietic progenitors reduces the risk of leukemogenic-process initiation from this compartment.
Definitive analysis of the DDR in the early stages of human hematopoiesis was unattainable, until recently, due to our inability to isolate the distinct, functionally homogeneous cellular subsets that make up the hematopoietic tissue hierarchy. Fortunately, in the last 5 years, significant advances in cell-surface marker characterization, multicolor flow cytometry and functional clonal assays, which efficiently distinguish different stem and progenitor subsets, have led to the establishment of a detailed hierarchical map of human HSCs and progenitors (3-5). For example, costaining of lineage-negative (Lin-) hematopoietic cells with a panel of monoclonal cell-surface antibodies has identified functionally distinct populations of candidate human long-term HSCs (LT-HSCs)-CD34+CD38-CD90+CD45RA-CD49f+-and various CPs that reside in the CD34+CD38+CD90- immunophenotype. The self-renewal potential of LT-HSCs is most often measured by their ability to sustain multilineage hematopoiesis for at least 16 weeks upon transplantation into a properly conditioned recipient, and to reinitiate hematopoiesis upon secondary transplantation. HSCs that can only sustain hematopoiesis transiently (8 weeks) are defined as short-term HSCs (ST-HSCs) (1). Although the basic roadmap of hematopoiesis is largely conserved between mice and humans, their vastly different body masses, life spans and environmental exposures have led to several important differences in hematopoiesis, including species-specific responses to DNA damage (10).
Today, scientists have the tools to address the role of single molecules and pathways in HSCs. As a result, it has become clear that DDR regulators themselves are the key players in HSC-specific processes such as self-renewal and multilineage differentiation. Several excellent reviews describing adult stem cells’ responses to DNA damage are available (7,11-15). In this review, we summarize the progress made in this rapidly growing field in the last several years, placing special emphasis on the human HSC.
Endogenous DNA-damage accrual limits HSC function
The most convincing evidence that unrepaired genomic damage results in (age-dependent) decreases in HSC regeneration is based on the careful analysis of hematopoiesis and HSC functionality in mice that are deficient in key DNA-repair genes. Loss of DNA-damage sensors (e.g., Atm, Atr), and insufficiency of DNA repair due to mutations in the homologous recombination (HR) (Brca2, Fancc, Fancd2), nonhomologous end-joining (NHEJ) [DNA-dependent protein kinase (Prkdc), Ku80, Lig4], Mismatch repair (MMR) (Msh2) and Nucleotide excision repair (NER) (Ercc1, Xpd) pathways largely resulted in a dramatic loss of repopulation capacity when mutant HSCs were competed against their wild-type counterparts in a BM repopulation assay (7,16-25). Interestingly, young mutant mice had normal numbers of LT-HSCs as defined by surface markers, suggesting that the reason for their functional decline was the inability to self-renew optimally under conditions of BM regeneration.
Importantly, Rossi and colleagues (19) reported greatly elevated numbers of γH2AX foci, a marker for DSBs and replication stress, specifically in LT-HSCs isolated from 122-week-old mice. Of note, ST-HSCs and CPs contained similarly low numbers of γH2AX foci when isolated from both young and old animals (19). Thus, mouse models overwhelmingly demonstrate the accumulation of endogenous DNA damage in the genome of LT-HSCs, which eventually impairs their self-renewal.
Recently, several groups have analyzed the relationships between genomic DNA damage and human HSC function. For example, Yahata and colleagues (26) quantitated γH2AX foci in purified human HSCs (defined as Lin-CD34+CD38-) isolated from umbilical cord blood, and the BM of elderly healthy individuals and patients that had undergone HSC transplant. They only found an age-dependent increase in γH2AX-positive cells in HSCs, whereas no difference was seen in the matched progenitor compartment (defined as Lin-CD34+CD38+). Importantly, they also found an inverse correlation between the number of γH2AX foci and transplantability of human HSCs using humanized mice. Serial transplantation experiments with cord blood cells also revealed that HSCs and not progenitors accumulate signs of DNA damage that most probably originate from ROS (26).
Studies from Weissman’s and Goodhardt’s groups analyzed age-dependent changes in human BM primitive hematopoietic subsets (LT-HSCs, Multipotential Progenitors and different CPs), as well as in their repopulation and differentiation capacities (27,28). Interestingly, both studies found accumulation of immunophenotypic HSCs with age, with no marked decline in their reconstitution potential, which stands in contrast to the mouse models of HSC aging. Unfortunately, no analysis of DNA damage-related markers was performed, warranting further exploration. In yet another study, CD34+CD38- BM cells isolated from healthy elderly (>70 years of age) individuals contained two times more γH2AX foci than the same cell subset obtained from cord blood (at birth). Of note, most of this physiological DNA damage did not colocalize with telomeres, which undergo an age-dependent decline in the same immunophenotype, arguing against telomeres as the origin of DNA damage in human primitive hematopoietic cells (29). An extreme example of the functional decline of human HSCs due to unrepaired genomic damage was recently provided by Ceccaldi and colleagues’ very thoughtful study (30) using FA syndrome-derived hematopoietic cells. First, they demonstrated loss of primitive hematopoietic cells (CD34+) before the onset of clinical BM failure. Equally important, they proved that the deficient FA HSCs and progenitors exhibit strong γH2AX positivity and become arrested in the G0/G1 state, thus preventing BM cell proliferation and leading to the clinical symptoms of the syndrome. Therefore, it is evident that human primitive hematopoietic cells are very sensitive to a congenital deficit in DNA repair, as in the case of FA that results in the early onset of BM failure as a result of HSC depletion and/or transformation toward Acute Myeloid Leukemia (AML). Furthermore, a gradual age-dependent increase in DNA alterations was reported in long-lived self-renewing HSCs based on next-generation sequencing analysis, supporting the notion that HSCs are reservoirs of both neutral and potentially dangerous mutations (8,9). Clearly, there must be a tradeoff between preservation of the HSC genome’s integrity and maintenance of a reasonable number of HSCs to ensure homeostasis. Now, we shall discuss the molecular mechanisms by which DNA-damage signals regulate HSC biology.
HSCs and DNA-repair pathways
The essential role of NER, HR, FA, NHEJ and MMR DNA-repair pathways in the regulation of murine HSC function is well documented and reviewed elsewhere (7,25). In contrast, the study of DNA repair in the human primitive hematopoietic compartment is still in its infancy. Recent studies from Dick’s laboratory in collaboration with the Mein group have provided first insight into the capacity for DNA DSB repair in human HSCs and CPs (31). To investigate this pathway researchers purified Lin-CD34+CD38-/lowCD45RA- cells (which are enriched in HSCs) and Lin-CD34+CD38+cells (contain only CPs and are unable to self-renew) from umbilical cord blood. Cells of both fractions were exposed to IR, and kinetics of DSB rejoining was measured using the neutral comet assay. Strikingly, no detectable rejoining of DSBs occurred in the first 30 min post-IR in the quiescent HSC-enriched fraction. In contrast, the CP fraction rejoined 20% of the breaks during the same time period (31). In agreement with these findings, Zhou and his group (32) found that CD34+CD38- cells isolated from human BM are less efficient in DSB rejoining than their more mature progeny (CD34+CD38+ cells). To make this observation, Shao et al. developed an in vitro cell-free assay for NHEJ based on linearized substrates to measure DNA repair (32).
The major pathway for DSB repair in quiescent human cells is NHEJ, a rapid but error-prone method that is responsible for most of the genomic structural variants and chromosomal translocations in human cancers (33). In mammalian cells, the protein components of NHEJ pathway begin to assemble at DSB sites seconds after IR, and NHEJ-mediated repair and rejoining of DSBs is detected within 10 min of DSB induction (34). In line with this rapid kinetics, ≈50% of DSBs are rejoined within 30 min post-IR by quiescent human fibroblasts (35). Therefore, the HSC-intrinsic “deficit” in NHEJ activity relative to progenitors may indicate that a distinct DDR begins in HSCs immediately post-IR. To explain this “deficit”, one can raise the hypothesis that human HSCs possess a DSB-repair machinery with higher stringency than that of their progeny to reduce the risk of mutagenesis. Alternatively, HSCs might be less efficient in DSB repair, which would explain the reported persistence of γH2AX foci (26,29,31). Future experiments should be aimed at revealing the molecular mechanism underlying the distinct dynamics of the DDR in human HSCs.
In contrast to the results obtained with human hematopoietic cells, highly purified mouse HSCs showed higher NHEJ activity than committed myeloid progenitors (32,36). Several important technical aspects should be considered before interpreting these potentially important interspecies differences. First, human HSC-containing populations are more heterogeneous than those of mice and additional human HSC-specific surface markers have recently been identified (3). Second, human cord blood and adult murine BM cells might represent developmentally incomparable HSC subsets. In this respect, the role of different niche factors should also be considered and will be discussed further on. Finally, although both human and mouse HSCs are confined to the G0 stage of the cell cycle, cord blood-derived progenitors are not cycling (no cells in the S/G2/M phases of the cell cycle). In contrast, BM progenitors are highly cycling, further complicating a direct comparison. Bearing in mind these important experimental caveats, all three studies point to the existence of distinct DNA-repair characteristics in HSCs from the two species.
Differences in the basal expression of DNA-repair proteins provide important correlative evidence for the observed differences in DNA-repair activity (Table 1). For example, murine HSCs exhibited increased expression of numerous transcripts encoding NHEJ proteins relative to their downstream progeny. The opposite pattern of expression was evident for HR-repair genes. This transcriptional analysis correlates well with the enhanced NHEJ activity of murine HSCs (32,36). Less is known about DNA-repair gene expression in human primitive HSCs (37,38). Intriguingly, lower levels of ATM and Lig4 mRNA were expressed in human HSCs than in the more mature cells (32). Whether this difference might account for the relatively less effective NHEJ in human HSCs remains to be established. It is important to bear in mind that the activity of some DNA-repair proteins is regulated post-transcriptionally and in a cell-cycle dependent manner. Furthermore, human cells typically express more Ku and DNA-dependent protein kinase (DNA-PK) proteins (discussed further on) than their murine counterparts, adding to the difference in cellular concentration and activity of repair proteins (39-41). Accurate analysis of DNA-repair protein levels and their biochemical activity in DNA-repair assays in the specific cellular contexts (HSCs and progenitors) will undoubtedly reveal the underlying mechanism and significance of the observed interspecies differences.
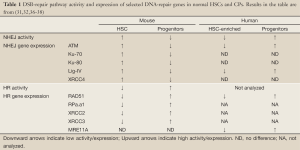
Full table
DDR signaling governs HSC functions
As already discussed, the accumulation of DNA damage in primitive hematopoietic cells threatens the organism’s health. Thus, a complex network of DDR factors that include sensors, regulators and effectors has evolved to respond effectively to genotoxic stress. Activation of this network results in two primary outcomes: repair of DNA damage and genome restoration or, if damaged DNA cannot be sufficiently repaired, execution of cell death, differentiation or senescence programs (41-43).
Of the many different lesions that occur in DNA molecules, DSBs induced by IR are the most lethal for the individual cells as well as for the whole hematopoietic tissue (44). This notion is exemplified by the dramatic loss of HSC self-renewal potential as measured by competitive repopulation or serial transplantation assays, upon exposure of the mouse to even relatively low doses of IR (2-3 Gy) (31,36,45). Thus, IR is the most frequently used trigger to provoke a DDR and delineate the molecular pathways engaged.
In response to DSBs, cells mount a powerful signaling network. It starts with rapid aggregation of multiple protein “sensors” around the DNA break, which engages the activation of several kinases termed “transducers”. These further amplify the DSB stress signal to numerous downstream effectors that ensure appropriate alterations in the cell’s fate (41-43). It is becoming increasingly clear that many of the central regulators of the DDR play a key role in the regulation of HSC homeostasis (13,14,25,46-49).
We will first discuss the mechanisms by which upstream DDR kinases participate in HSC maintenance. Then we will integrate the tumor suppressor p53-a prototype DDR effector, and we will finish by highlighting the role of “bona fide” apoptosis and differentiation genes that become co-opted by the complex genetic network regulating HSC function under both homeostatic and stress conditions.
The upstream kinases
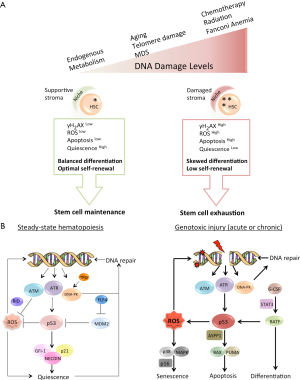
The protein kinase ATM is the primary transducer of the DSB signal via phosphorylation of the numerous nuclear and cytoplasmic substrates that participate in DNA repair, apoptosis and senescence. A detailed analysis of HSC function in ATM-deficient mice by Ito and colleagues (21,47) revealed that ATM is essential to maintaining HSC regeneration, whereas it is less important for the proliferation or differentiation of CPs (21,50). Even without exogenous stress, ATM-/- animals exhibit age-dependent BM failure, due to the mutant HSC’s inability to self-renew. Elevated levels of ROS were found in different primitive BM cells in ATM-/- mice as compared to their normal littermates, consistent with the previously described role of ATM in regulating cellular antioxidative systems (51). Crucially, LT-HSCs, in contrast to progenitors, were found to be most vulnerable to the elevated ROS levels that induced loss of HSC quiescence, increased apoptosis and diminished self-renewal. Mechanistically, HSC-specific activation of p38 mitogen-activated protein kinase (p38 MAPK) induced by ROS in ATM-/- is responsible for the loss of HSC function. These connections were proven by rescue of BM failure and restoration of HSC self-renewal by treating ATM-deficient mice with a small-molecule inhibitor of p38 MAPK or with the antioxidant N-acetyl-L-cysteine (NAC) (21,50) (Figure 1). A recent study from Gross’s laboratory (52) provided evidence for ATM-dependent phosphorylation of BID, a BH3-only pro-death Bcl-2 family member, determining the level of ROS in murine HSCs. HSCs isolated from mice in which the ATM-phosphorylation site on the BID protein was mutated (BIDAA) had elevated ROS levels, decreased quiescence, and inferior competitive repopulation potential. As in the case of ATM-knockout mice, antioxidant treatment returned HSCs to the quiescent state and restored their repopulation potential. Importantly, both human and mouse HSCs accumulated ROS during serial transplantation assay in vivo and increased ROS levels coincided with decreased HSC self-renewal in both species (26,53). Therefore, ROS levels inside HSCs, governed in part by the ATM-BID axis, control HSC self-renewal potential (Figure 1).
In addition to ATM, DSBs activate the DNA-PK complex which consists of the catalytic subunit (DNA-PKcs) and the DNA-binding Ku70/80 heterodimer. The DNA-PK complex recognizes DSBs and its function is essential for the NHEJ-repair pathway. Consistent with this notion, BM from Ku80-/- and DNA-PK-/- animals exhibited progressive loss of regeneration potential due to a decrease in HSC self-renewal (19,23). Two recent works have described HSC-specific regulation of DNA-PK function and its importance in HSC biology (23,54). To investigate the physiological significance of IR-induced DNA-PK phosphorylation, Zhang and colleagues generated a DNA-PK3A/3A mouse in which threonine residues that are subject to IR-induced phosphorylation (Thr 2643, 2634 and 2605) are replaced with alanine (23). In striking contrast to the modest HSC dysfunction observed in aged DNA-PKcs-knockout mice, DNA-PKcs3A/3A mice developed congenital hematopoietic failure caused by rapid demise of fetal liver HSCs by p53-dependent apoptosis. Compound deficiency in NHEJ as well as in the HR and FA DNA-repair pathways observed in DNA-PKcs3A/3A-derived fibroblasts can explain the severity of the hematopoietic phenotype (23).
Availability of DNA repair factors in HSC and early progenitors is important for the efficient DDR and preservation of genome stability. Indeed, many DNA repair factors are differentially expressed between HSC and different committed progenitor populations (32,36-38) (Table 1). However, the molecular circuitry that governs DNA repair gene expression in primitive hematopoietic cells is not well understood. Recently, Zheng and colleagues uncovered that mTOR kinase is required to maintain basal levels of FANCD2 in murine HSC, progenitors and human hematopoietic cells (55). Intriguingly, overactivation of mTOR, caused by PTEN deletion, results in normal HSC depletion and accelerated leukemogenesis (56). How mTOR-dependent regulation of FA repair pathway contributes to its pleiotropic roles in carcinogenesis should be determined in the future.
How DNA repair in primitive hematopoietic cells is regulated by the microenvironment is not entirely known. Recently, de Laval and colleagues (54) found that thrombopoietin (TPO) and its receptor MPL, previously implicated in maintenance of HSC quiescence and self-renewal, are also important for DSB repair in mouse and human primitive hematopoietic cells. TPO/MPL-signaling stimulated DNA-PK-dependent NHEJ efficiency by a significant increase in Ser 2056 and Thr 2609 phosphorylation on DNA-PK. Intriguingly, Thr 2609 in human DNA-PK corresponds to Thr 2605 in the murine protein and, if not properly modified by the TPO/MPL axis, might contribute to HSC ablation as observed in the DNA-PKcs3A/3A model discussed above. This study highlights previously unrecognized crosstalk between a niche factor, such as TPO, and regulation of DNA repair in HSCs (Figure 1B). It is likely that more microenvironmental cues governing the HSC-specific DDR will be described in the future.
p53: a double-edged sword in HSC regeneration
The tumor suppressor p53 is a widely expressed transcription factor that plays a critical role in regulating several DDR pathways, including activation of cell-cycle arrest, induction of apoptosis, senescence, suppression of HR, and others (57). As with additional DDR genes, germline mutations in p53 increase the incidence of hematological malignancy, and somatic aberrations of p53 are frequent in leukemia, lymphoma and myelodysplastic syndrome (58,59). The importance of p53 in IR-induced apoptosis has been analyzed in detail both in vitro and in vivo and attributed mainly to its transcriptional target Puma (60) (Figure 1B).
However, it has been difficult to establish the function of p53 in HSCs compared to other hematopoietic cells using mouse models. BM progenitors from p53-knockout mice exhibit increased resistance to DNA damage-induced apoptosis in short-term survival assays (36,60-64). Still, when wild-type murine LT-HSCs defined as Lin-Sca1+Kit+Flk2- cells were exposed to 2 or 5.5 Gy of IR, no increase in cleaved caspase-3, a marker of apoptosis, was observed at 6–24 h post-damage. At the same time, 20-60% of Common Myeloid and Granulocyte-Macrophage Progenitors underwent p53-dependent apoptosis (36,64). These observations would suggest little involvement of p53-dependent apoptosis in the irradiated murine LT-HSCs. However, meticulous analysis of BM cellularity under the even lower IR dose of 1.75 Gy revealed a dramatic, time-dependent loss of LT-HSCs cells as measured up to 7 days post-damage. Analysis of cell death by Annexin V staining clearly implicated apoptosis as the reason for HSC loss (65-67). Of note, wild-type LSK cells exhibited persistent apoptosis even 7 days post-IR (65). No change in cell numbers in the Annexin-positive cell fraction was found in the BM of similarly irradiated p53-/- and Puma-/- animals (49,65,68). Although the reason for this persistence of apoptosis was not examined in those studies, Wang et al. (69) found a sustained increase in ROS production and subsequently higher oxidative DNA damage in HSCs isolated from mice exposed to sublethal total body irradiation. Based on these pieces of evidence, murine LT-HSCs are very sensitive to IR-induced apoptosis mediated by p53 via its transcriptional target Puma. It is likely that the different apoptosis assays (cleaved caspase-3 vs. Annexin) and variability in the time points after IR (hours vs. days) employed by the different groups led to the apparently conflicting conclusions.
When human primitive hematopoietic cells derived from cord blood were exposed to 3 Gy of IR, the HSC-enriched fraction underwent significantly higher cell death relative to the downstream CPs in both short-term and long-term viability assays (31). Preferential expression of p53 pro-apoptotic partner ASPP1 in the HSCs was partially responsible for their enhanced sensitivity to p53-mediated apoptosis upon IR. Increased resistance of human HSCs and progenitors to apoptosis accounts for the enhanced hematopoietic regeneration when irradiated HSCs in which p53 had been disabled were transplanted into conditioned recipients (31). Thus, both phenotypic and functional assays indicate that acute genotoxic injury initiates a p53-dependent apoptosis pathway that ablates primitive hematopoietic cells in both humans and mice.
Rapid transformation of BM cells isolated from p53-/- mice complicates the analysis of p53 function in normal non-malignant repopulating LT-HSCs, and precludes an examination of their self-renewal by retransplantation (70,71). When attempts were made to examine the function of putatively non-malignant HSCs from p53-deficient mice, once again, conflicting results were obtained. Although there were differences in the designs of the competitive repopulation assays, it was suggested that HSC repopulation increased, remained the same or even decreased (70,72-74). However, when the dosage of p53 was increased, HSCs exhibited reduced competitive repopulation potential (71).
Studies of human HSCs in which p53 activity had been disabled by dominant negative mutation or by means of RNA interference extended observations made in mice and provided some clarification of the role of p53 in the regulation of HSC self-renewal. Head-to-head analysis of human HSCs in which p53-dependent apoptosis was bypassed by Bcl-2 overexpression established that p53 has both positive and negative roles in the regulation of HSC function. Human HSCs with inactive p53 demonstrated increased numbers of γH2AX foci in secondary transplants as compared with control and Bcl-2-overexpressing HSCs (31). Once again, engagement of the DDR machinery in HSCs resulted in reduced HSC self-renewal. Apoptosis-independent p53 effectors, such as p21 and others, might participate in HSC genome quality control coupled with self-renewal (64). In addition, positive regulation of HSC quiescence by p53 via its transcriptional targets Gfi-1 and Necdin may well contribute to the role of p53 in preserving LT-HSC function. Furthermore, fine regulation of p53 activity by Elf4/Mdm2 factors was also implicated in the maintenance of HSC quiescence (72) (Figure 1B).
Thus, p53 plays at least two distinct physiological roles to ensure optimal HSC function: apoptosis regulation and prevention of genomic damage accumulation upon HSC self-renewal. The complex network of p53 transcriptional targets and cofactors involved in the regulation of HSC quiescence, cell-cycle arrest, apoptosis and senescence determines HSC fate based on the severity and persistence of the genotoxic stress (Figure 1).
DDR-dependent HSC differentiation checkpoint
A higher number of γH2AX foci, shortened telomeres and elevated ROS levels characterize HSCs isolated from elderly humans and mice. In both species, hematopoietic stem and progenitor cells undergo alterations in lineage distribution, gene expression, epigenetics, differentiation potential and repopulation capacity (27-29,75). Decreased lymphoid cell output by aged HSCs represents one of the best-documented functional changes (76,77). An intriguing connection between the DNA-damage signal and augmented lymphoid differentiation of murine HSCs was recently provided by Rudolph’s laboratory. In an elegant in-vivo shRNA screen, Wang et al. (78) identified transcription factor BATF as the major culprit limiting self-renewal of HSCs with dysfunctional telomeres. IR or critically short telomeres induced an increase in BATF expression in HSCs via a G-CSF- and STAT3-dependent mechanism. Then, BATF specifically engaged the lymphoid differentiation program among CD150+/low HSCs, thus limiting their self-renewal potential. Intriguingly, DNA damage-induced accumulation of activated p53 (P-Ser15 p53) and of its downstream target p21 was abrogated in the BM cells expressing shBATF, positioning BATF upstream of p53 in the DDR-signaling cascade. The direct correlation between BATF mRNA level and p21 expression documented in CD34+ cells derived from patients with preleukemic syndrome (MDS) warrants further investigation of BATF function in human leukemogenesis. In summary, the HSC differentiation checkpoint mediated by BATF upon DNA damage represents a failsafe mechanism that culls HSCs with accrued genomic aberrations. In concert with apoptosis, this novel differentiation checkpoint might explain the aging-associated decline in HSC function and these cells’ unbalanced differentiation. Importantly, the ability of DNA damage to induce premature stem cells differentiation was also documented in another stem cell compartment in the mouse, namely, Melanocyte Stem Cells (79). In this case, IR triggered p53 and INK4A independent loss of Melanocyte Stem Cells self-renewal that led to the appearance of ectopically pigmented melanocytes and hair graying of the irradiated animals. Interestingly, murine Bulge Stem Cells that share with Melanocyte Stem Cells the same niche turned to be resistant to both irradiation induced apoptosis and differentiation (80) pointing to the distinct pathways regulating various tissue stem cells response to DNA damage.
Summary and future directions
An efficient DDR is one of the major evolutionary adaptations of all living organisms, ensuring the transfer of accurate genomic information from one generation to the next. Research into the DDR in the last decades has identified hundreds of proteins that form myriad functional intracellular interactions to accomplish various tasks in the injured cell, including DNA repair, cell-cycle arrest, apoptosis and others. In the context of long-lived animals, such as humans and rodents, causative relationships between the DDR, tissue regeneration, organismal aging and cancer susceptibility become established. Much of this important knowledge is derived from the study of DDR in the context of hematopoiesis-a vital process sustained by a small fraction of quiescent self-renewing stem cells (Figure 1A). Understanding the HSC-specific DDR has revealed important principles employed by self-renewing tissues to withstand DNA damage. Some generalizations can be made based on the available experimental evidence as outlined in this review. First, HSCs are selectively sensitive to acute and chronic genome damage, which results in a dramatic reduction in their regenerative potential. Second, elevated ROS levels and subsequent oxidative DNA damage inside HSCs are common motifs in the HSC DDR. Accordingly, antioxidant treatment is effective in reversing the ROS-induced decline in HSC self-renewal. Third, the quiescent state forces HSCs to employ the error-prone NHEJ for DSB repair. Finally, multiple genes involved in DDR pathways also regulate HSC self-renewal (Figure 1B). The critical differences in DDR between humans and rodents at the level of HSCs, highlighted in this review, preclude direct extrapolation of findings from one species to the other (Table 1). Although the driving force for the described interspecies differences is currently unknown, it is tempting to speculate that vastly different lifespan between the two species had major impact on their respective environmental adaptations, including different strategies to deal with DNA damage.
Obviously, exciting discoveries made in the last few years have presented the scientific community with even more burning questions. For instance, why is leukemia a rare disease given that 1011 new blood cells are produced daily? Do human and rodent HSCs employ opposite strategies to deal with DNA damage? If so, what is the molecular basis and physiological significance of this interspecies difference? Is it possible to manipulate the DDR to improve HSC fitness and delay aging of self-renewing cells? What are additional intrinsic and microenvironment-derived regulators of the DDR in HSCs? Will therapeutic targeting of DDR effectors that regulate self-renewal selectively eliminate leukemia stem cells and spare normal HSCs? The very important question asked by Shakespeare’s hero, without thinking of HSC-specific DDR: “To be, or not to be…” will undoubtedly keep us busy for the foreseeable future.
Acknowledgments
The authors apologize to their colleagues whose important work was not cited due to space limitations.
Funding: This work was supported by funds from the Israel Cancer Association (M.M.) and FP7-PEOPLE-2012-CIG (M.M.).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Daohong Zhou and Chuan-Yuan Li) for the series “Stem Cells in Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.09.02). The series “Stem Cells in Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Doulatov S, Notta F, Laurenti E, et al. Hematopoiesis: a human perspective. Cell Stem Cell 2012;10:120-36. [PubMed]
- Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell 2008;132:681-96. [PubMed]
- Notta F, Doulatov S, Laurenti E, et al. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science 2011;333:218-21. [PubMed]
- Majeti R, Park CY, Weissman IL. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell 2007;1:635-45. [PubMed]
- Doulatov S, Notta F, Eppert K, et al. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat Immunol 2010;11:585-93. [PubMed]
- Barnes DE, Lindahl T. Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu Rev Genet 2004;38:445-76. [PubMed]
- Niedernhofer LJ. DNA repair is crucial for maintaining hematopoietic stem cell function. DNA Repair (Amst) 2008;7:523-9. [PubMed]
- Welch JS, Ley TJ, Link DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell 2012;150:264-78. [PubMed]
- Jan M, Snyder TM, Corces-Zimmerman MR, et al. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci Transl Med 2012;4:149ra118.
- Parekh C, Crooks GM. Critical differences in hematopoiesis and lymphoid development between humans and mice. J Clin Immunol 2013;33:711-5. [PubMed]
- Mandal PK, Blanpain C, Rossi DJ. DNA damage response in adult stem cells: pathways and consequences. Nat Rev Mol Cell Biol 2011;12:198-202. [PubMed]
- Sperka T, Wang J, Rudolph KL. DNA damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol Cell Biol 2012;13:579-90. [PubMed]
- Naka K, Hirao A. Maintenance of genomic integrity in hematopoietic stem cells. Int J Hematol 2011;93:434-9. [PubMed]
- Asai T, Liu Y, Bae N, et al. The p53 tumor suppressor protein regulates hematopoietic stem cell fate. J Cell Physiol 2011;226:2215-21. [PubMed]
- Abbas HA, Pant V, Lozano G. The ups and downs of p53 regulation in hematopoietic stem cells. Cell Cycle 2011;10:3257-62. [PubMed]
- Cho JS, Kook SH, Robinson AR, et al. Cell autonomous and nonautonomous mechanisms drive hematopoietic stem/progenitor cell loss in the absence of DNA repair. Stem Cells 2013;31:511-25. [PubMed]
- Garaycoechea JI, Crossan GP, Langevin F, et al. Genotoxic consequences of endogenous aldehydes on mouse haematopoietic stem cell function. Nature 2012;489:571-5. [PubMed]
- Langevin F, Crossan GP, Rosado IV, et al. Fancd2 counteracts the toxic effects of naturally produced aldehydes in mice. Nature 2011;475:53-8. [PubMed]
- Rossi DJ, Bryder D, Seita J, et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 2007;447:725-9. [PubMed]
- Nijnik A, Woodbine L, Marchetti C, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature 2007;447:686-90. [PubMed]
- Ito K, Hirao A, Arai F, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 2004;431:997-1002. [PubMed]
- Chen M, Tomkins DJ, Auerbach W, et al. Inactivation of Fac in mice produces inducible chromosomal instability and reduced fertility reminiscent of Fanconi anaemia. Nat Genet 1996;12:448-51. [PubMed]
- Zhang S, Yajima H, Huynh H, et al. Congenital bone marrow failure in DNA-PKcs mutant mice associated with deficiencies in DNA repair. J Cell Biol 2011;193:295-305. [PubMed]
- Reese JS, Liu L, Gerson SL. Repopulating defect of mismatch repair-deficient hematopoietic stem cells. Blood 2003;102:1626-33. [PubMed]
- Kenyon J, Gerson SL. The role of DNA damage repair in aging of adult stem cells. Nucleic Acids Res 2007;35:7557-65. [PubMed]
- Yahata T, Takanashi T, Muguruma Y, et al. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood 2011;118:2941-50. [PubMed]
- Kuranda K, Vargaftig J, de la Rochere P, et al. Age-related changes in human hematopoietic stem/progenitor cells. Aging Cell 2011;10:542-6. [PubMed]
- Pang WW, Price EA, Sahoo D, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A 2011;108:20012-7. [PubMed]
- Rübe CE, Fricke A, Widmann TA, et al. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PLoS One 2011;6:e17487 [PubMed]
- Ceccaldi R, Parmar K, Mouly E, et al. Bone marrow failure in Fanconi anemia is triggered by an exacerbated p53/p21 DNA damage response that impairs hematopoietic stem and progenitor cells. Cell Stem Cell 2012;11:36-49. [PubMed]
- Milyavsky M, Gan OI, Trottier M, et al. A distinctive DNA damage response in human hematopoietic stem cells reveals an apoptosis-independent role for p53 in self-renewal. Cell Stem Cell 2010;7:186-97. [PubMed]
- Shao L, Feng W, Lee KJ, et al. A sensitive and quantitative polymerase chain reaction-based cell free in vitro non-homologous end joining assay for hematopoietic stem cells. PLoS One 2012;7:e33499 [PubMed]
- Raphael BJ, Volik S, Yu P, et al. A sequence-based survey of the complex structural organization of tumor genomes. Genome Biol 2008;9:R59. [PubMed]
- Gulston M, de Lara C, Jenner T, et al. Processing of clustered DNA damage generates additional double-strand breaks in mammalian cells post-irradiation. Nucleic Acids Res 2004;32:1602-9. [PubMed]
- Stenerlöw B, Karlsson KH, Cooper B, et al. Measurement of prompt DNA double-strand breaks in mammalian cells without including heat-labile sites: results for cells deficient in nonhomologous end joining. Radiat Res 2003;159:502-10. [PubMed]
- Mohrin M, Bourke E, Alexander D, et al. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell 2010;7:174-85. [PubMed]
- Bracker TU, Giebel B, Spanholtz J, et al. Stringent regulation of DNA repair during human hematopoietic differentiation: a gene expression and functional analysis. Stem Cells 2006;24:722-30. [PubMed]
- Buschfort-Papewalis C, Moritz T, Liedert B, et al. Down-regulation of DNA repair in human CD34(+) progenitor cells corresponds to increased drug sensitivity and apoptotic response. Blood 2002;100:845-53. [PubMed]
- Branzei D, Foiani M. Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol 2008;9:297-308. [PubMed]
- Chapman JR, Taylor MR, Boulton SJ. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell 2012;47:497-510. [PubMed]
- Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell 2010;40:179-204. [PubMed]
- Hiom K. Coping with DNA double strand breaks. DNA Repair (Amst) 2010;9:1256-63. [PubMed]
- Lukas J, Lukas C, Bartek J. More than just a focus: The chromatin response to DNA damage and its role in genome integrity maintenance. Nat Cell Biol 2011;13:1161-9. [PubMed]
- Gudkov AV, Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer 2003;3:117-29. [PubMed]
- Wang Y, Schulte BA, LaRue AC, et al. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood 2006;107:358-66. [PubMed]
- Schoppy DW, Ruzankina Y, Brown EJ. Removing all obstacles: a critical role for p53 in promoting tissue renewal. Cell Cycle 2010;9:1313-9. [PubMed]
- Abbas HA, Maccio DR, Coskun S, et al. Mdm2 is required for survival of hematopoietic stem cells/progenitors via dampening of ROS-induced p53 activity. Cell Stem Cell 2010;7:606-17. [PubMed]
- Valente LJ, Gray DH, Michalak EM, et al. p53 efficiently suppresses tumor development in the complete absence of its cell-cycle inhibitory and proapoptotic effectors p21, Puma, and Noxa. Cell Rep 2013;3:1339-45. [PubMed]
- Shao L, Sun Y, Zhang Z, et al. Deletion of proapoptotic Puma selectively protects hematopoietic stem and progenitor cells against high-dose radiation. Blood 2010;115:4707-14. [PubMed]
- Ito K, Takubo K, Arai F, et al. Regulation of reactive oxygen species by Atm is essential for proper response to DNA double-strand breaks in lymphocytes. J Immunol 2007;178:103-10. [PubMed]
- Barzilai A, Rotman G, Shiloh Y. ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair (Amst) 2002;1:3-25. [PubMed]
- Maryanovich M, Oberkovitz G, Niv H, et al. The ATM-BID pathway regulates quiescence and survival of haematopoietic stem cells. Nat Cell Biol 2012;14:535-41. [PubMed]
- Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 2007;110:3056-63. [PubMed]
- de Laval B, Pawlikowska P, Petit-Cocault L, et al. Thrombopoietin-increased DNA-PK-dependent DNA repair limits hematopoietic stem and progenitor cell mutagenesis in response to DNA damage. Cell Stem Cell 2013;12:37-48. [PubMed]
- Guo F, Li J, Du W, et al. mTOR regulates DNA damage response through NF-κB-mediated FANCD2 pathway in hematopoietic cells. Leukemia 2013;27:2040-6. [PubMed]
- Yilmaz OH, Valdez R, Theisen BK, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 2006;441:475-82. [PubMed]
- Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell 2009;137:413-31. [PubMed]
- Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 2013;368:2059-74. [PubMed]
- Prokocimer M, Rotter V. Structure and function of p53 in normal cells and their aberrations in cancer cells: projection on the hematologic cell lineages. Blood 1994;84:2391-411. [PubMed]
- Michalak EM, Villunger A, Adams JM, et al. In several cell types tumour suppressor p53 induces apoptosis largely via Puma but Noxa can contribute. Cell Death Differ 2008;15:1019-29. [PubMed]
- Wu WS, Heinrichs S, Xu D, et al. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell 2005;123:641-53. [PubMed]
- Lotem J, Sachs L. Hematopoietic cells from mice deficient in wild-type p53 are more resistant to induction of apoptosis by some agents. Blood 1993;82:1092-6. [PubMed]
- Na Nakorn T, Traver D, Weissman IL, et al. Myeloerythroid-restricted progenitors are sufficient to confer radioprotection and provide the majority of day 8 CFU-S. J Clin Invest 2002;109:1579-85. [PubMed]
- Insinga A, Cicalese A, Faretta M, et al. DNA damage in stem cells activates p21, inhibits p53, and induces symmetric self-renewing divisions. Proc Natl Acad Sci U S A 2013;110:3931-6. [PubMed]
- Labi V, Villunger A. PUMA-mediated tumor suppression: a tale of two stories. Cell Cycle 2010;9:4269-75. [PubMed]
- Labi V, Erlacher M, Krumschnabel G, et al. Apoptosis of leukocytes triggered by acute DNA damage promotes lymphoma formation. Genes Dev 2010;24:1602-7. [PubMed]
- Michalak EM, Vandenberg CJ, Delbridge AR, et al. Apoptosis-promoted tumorigenesis: gamma-irradiation-induced thymic lymphomagenesis requires Puma-driven leukocyte death. Genes Dev 2010;24:1608-13. [PubMed]
- Yu H, Shen H, Yuan Y, et al. Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose gamma-irradiation. Blood 2010;115:3472-80. [PubMed]
- Wang Y, Liu L, Pazhanisamy SK, et al. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic Biol Med 2010;48:348-56. [PubMed]
- TeKippe M, Harrison DE, Chen J. Expansion of hematopoietic stem cell phenotype and activity in Trp53-null mice. Exp Hematol 2003;31:521-7. [PubMed]
- Dumble M, Moore L, Chambers SM, et al. The impact of altered p53 dosage on hematopoietic stem cell dynamics during aging. Blood 2007;109:1736-42. [PubMed]
- Liu Y, Elf SE, Miyata Y, et al. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell 2009;4:37-48. [PubMed]
- Marusyk A, Porter CC, Zaberezhnyy V, et al. Irradiation selects for p53-deficient hematopoietic progenitors. PLoS Biol 2010;8:e1000324 [PubMed]
- Chen J, Ellison FM, Keyvanfar K, et al. Enrichment of hematopoietic stem cells with SLAM and LSK markers for the detection of hematopoietic stem cell function in normal and Trp53 null mice. Exp Hematol 2008;36:1236-43. [PubMed]
- Chen J. Hematopoietic stem cell development, aging and functional failure. Int J Hematol 2011;94:3-10. [PubMed]
- Chambers SM, Shaw CA, Gatza C, et al. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol 2007;5:e201 [PubMed]
- Rossi DJ, Bryder D, Zahn JM, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A 2005;102:9194-9. [PubMed]
- Wang J, Sun Q, Morita Y, et al. A differentiation checkpoint limits hematopoietic stem cell self-renewal in response to DNA damage. Cell 2012;148:1001-14. [PubMed]
- Inomata K, Aoto T, Binh NT, et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell 2009;137:1088-99. [PubMed]
- Sotiropoulou PA, Candi A, Mascré G, et al. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat Cell Biol 2010;12:572-82. [PubMed]


