
Have we considered all barriers to mammalian target of rapamycin inhibition as treatment for diffuse intrinsic pontine glioma?
Diffuse intrinsic pontine glioma (DIPG) is a uniformly lethal primary pediatric brain tumor (1,2). Surgical resection is impossible due to its location in the brainstem and invasive nature. Radiotherapy provides just a minor incremental extension in overall survival, whereas chemotherapy is largely ineffective (3-5). There is thus a high unmet need for better therapies. Importantly, several recent studies have characterized the genomic landscape of DIPG, revealing common genetic alterations in ACVR1, histone H3, ATRX and TP53 (6-9). Unfortunately, these are mostly not yet appreciable druggable targets. Next to these alterations, a fraction of DIPGs is characterized by increased receptor tyrosine kinase-RAS-PI3K-AKT signaling, e.g., as a result of AKT gain or phosphatase and tensin homolog (PTEN) loss, making this pathway a potentially attractive target for therapy (10,11). Researchers from the lab of Dr. Raabe at Johns Hopkins University School of Medicine in Baltimore have therefore investigated targeting the PI3K-AKT-mTOR pathway as a potential therapeutic strategy for DIPG. In a recent study in Cancer Letters, Miyahara and colleagues demonstrate that the mammalian target of rapamycin complex 1 and 2 (mTORC1/2) inhibitor TAK228 (INK128, sapanisertib) delayed tumor formation of an orthotopic murine model of DIPG (12) and conclude that mTOR inhibition may be a promising therapeutic strategy for treatment of DIPG.
The authors first demonstrate that TAK228 dose-dependently inhibits signaling through the PI3K-AKT-mTOR pathway and proliferation in three independent patient-derived spheroid DIPG cell lines, requiring continuous exposure to drug concentrations of about 5–25 nM. Secondly, they show that 25 nM of drug reduces the fraction of proliferating bromodeoxyuridine (BrdU) positive cells from 44–50% to 32–37% and find an additive reduction to 21–26% when combined with 2 Gy of radiation. These findings correlate with the increased fraction of cleaved caspase 3 (CC3) positive cells, a commonly used marker for apoptosis. In two of the three cell lines, this increase in CC3 positive cells was associated with reduced expression of the anti-apoptotic proteins BCL-2 and BCL-XL. Third, the authors show that TAK228 can suppress the migration/invasion of DIPG cells using a transwell assay in which DIPG cells are seeded onto matrigel coated inserts and allowed to migrate into a compartment containing either drug-free neurobasal medium with growth factors or the same medium containing 10 nM of TAK228.
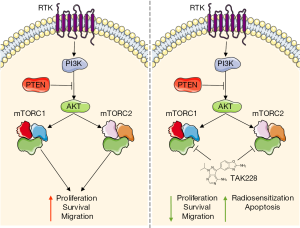
Finally, the authors confirm that the previous findings on cell signaling, proliferation, increase in CC3 positive cells and reduction of migration (by scratch assay) also occur in a cell line that was derived from a genetically engineered murine PDGF-B; H3.3-K27M; p53–/– DIPG model (13). Subsequently, the authors use this cell line to validate their in vitro findings in an in vivo orthotopic DIPG xenograft model. They inject murine DIPG neurospheres in the brainstem of 12 NOD/SCID gamma mice and randomize the animals one week after tumor cell injection between vehicle control treatment and 1 mg/kg/day of TAK228 in a 5-day on/2-day off schedule for the duration of the study. They demonstrate that TAK228 significantly delayed DIPG tumor formation, as indicated by increased survival. Taken together, the authors conclude from these experiments that mTOR inhibition is a promising strategy for DIPG treatment since it reduces proliferation, survival and migration and increases radiosensitivity and apoptosis (Figure 1) and suggest that a phase I study with TAK228 in pediatric brain tumor patients may be reasonable.
As outlined above, the introduction of more effective weaponries for the treatment of DIPG would be very much welcomed and the authors of this paper do their best to convince us about the potential of TAK228. However, we are of course also very much aware of the fact that DIPG is a tough adversary and that thus far all previous attempts with drugs have failed. What are the odds that TAK228 can indeed be a game changer in DIPG treatment or that it may be the next disappointment? In order to address this issue, let’s zoom in on a few critical issues.
PI3K-AKT-mTOR: driver or hitch-hiker?
In order to be a useful target for therapy, the tumor must have a dependency on a target to sustain. As mentioned above, the picture of the genetic landscape of DIPG is gaining more and more detail. The assumption of the authors that the AKT pathway is activated in approximately 70% of all DIPG patients is based on older publications from 2010 and 2012, but based on four more recent Nature Genetics papers from 2014 that describe the genomic landscape of DIPG (6-9), this is closer to 25%. Moreover, the PI3K-mTOR axis is one of the most frequently hijacked pathways in cancer, but this does not make all these cancers uniformly responsive to inhibitors. Importantly, a recent large scale molecular meta-analysis of DIPGs demonstrated that activating PI3K pathway mutations are randomly distributed amongst Histone-defined subgroups and do not define a patient subgroup with a distinctly different survival, begging the question whether this pathway is a driver or a hitch-hiker in DIPG (14).
Pharmacokinetic considerations
TAK228, formerly known as MNL0128 or INK128, is an orally bioavailable small molecule drug initially developed by Intellikine (15). The IC50 of TAK228 for mTORC1/2 is about 1 nM in cell-free systems, but as shown in this and other papers 10 to 25 nM is generally required for inhibition of cell proliferation. Preclinical data on pharmacokinetics is relatively scarce, but Hsieh et al. have documented that the plasma Cmax level of TAK228 is 500 nM when given orally at a dose of 1 mg/kg. Thus, potentially active drug levels can be achieved in vivo at tolerable dose levels, explaining the efficacy observed in preclinical models. However, a recent Phase I study in patients shows that the plasma levels at the MTD of 4 mg/day does not exceed 25 ng/mL (80 nM), rendering this potential therapeutic window considerably smaller in humans (16). Thus, whereas inadequate exposure may already become a critical issue in all tumor types, it will be even more so in DIPG. DIPGs are notorious for their drug inaccessibility because of their location behind a more or less intact blood-brain barrier (BBB), as indicated by heterogeneous gadolinium enhancement on magnetic resonance imaging (MRI) (17). Nothing has yet been reported on the BBB permeability of TAK228 and although it is a relatively small molecule drug (MW 309.3), this does not automatically warrant good brain distribution.
Efficacy model considerations
The authors show in vivo efficacy against an orthotopic DIPG model using a dose of 1 mg/kg/day given Monday-through-Friday. Unfortunately, the authors have restricted in vivo efficacy testing to one murine DIPG model that is driven by platelet-derived growth factor B (PDGF-B) overexpression. This model is a genetically engineered PDGF-B; H3.3-K27M; p53–/– model referred to as the “PKC model”. Although overexpression of the PDGF-A receptor is a relatively common event in DIPG, the urgent question is how representative a PDGF-B driven model is for human DIPG. Clearly, PDGF-B is a potent oncogenic driver that relays proliferation via PI3K-mTOR, thus creating a major dependency on mTOR (18,19). Moreover, pericytes that intimately surround the brain endothelial cells are rich in PDGF-receptor and the continuous activation of these pericytes by tumor cell-produced PDGF-B results in a very leaky BBB that is typical for PDFG-B driven models (20-22). Interestingly, PDFG-B has also been shown to induce secretion of vascular endothelial growth factor (VEGF) in various cell types, possibly further contributing to a leaky vasculature (18,23). Leakiness of this particular PKC model was also previously shown in a paper demonstrating that the level of the kinase inhibitor BMS-754807 is approximately 3-fold higher in brain tumor tissue compared to normal brain (24). Thus, the authors’ model-of-choice combines two features that are unusual for DIPG: dependency on mTOR and a highly permeable BBB. Notably, these authors do have potentially more relevant patient-derived DIPG models available (25-27). Their main motivation for using the murine PKC model is the long latency of tumor formation of the human DIPG cell lines. Although practical considerations certainly have a place in science, we do feel that in this case these arguments do not outweigh the requirement of using biologically relevant preclinical tumor models.
To summarize, DIPG presents a formidable opponent and we can only applaud the authors’ ventures to explore novel therapeutic avenues to tackle this devastating disease. However, to our opinion the present preclinical work with TAK228 is not yet convincing enough to support its further clinical development in DIPG.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Ning Huang (Department of Neurosurgery, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.10.38). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Warren KE. Diffuse intrinsic pontine glioma: poised for progress. Front Oncol 2012;2:205. [Crossref] [PubMed]
- Jones C, Karajannis MA, Jones DT, et al. Pediatric high-grade glioma: biologically and clinically in need of new thinking. Neuro Oncol 2017;19:153-61. [PubMed]
- Packer RJ, Boyett JM, Zimmerman RA, et al. Hyperfractionated radiation therapy (72 Gy) for children with brain stem gliomas A childrens cancer group phase I/II trial. Cancer 1993;72:1414-21. [Crossref] [PubMed]
- Jennings MT, Sposto R, Boyett JM, et al. Preradiation chemotherapy in primary high-risk brainstem tumors: phase ii study CCG-9941 of the Children’s cancer group. J Clin Oncol 2002;20:3431-7. [Crossref] [PubMed]
- Cohen KJ, Heideman RL, Zhou T, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children's Oncology Group. Neuro Oncol 2011;13:410-6. [Crossref] [PubMed]
- Taylor KR, Mackay A, Truffaux N, et al. Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat Genet 2014;46:457-61. [Crossref] [PubMed]
- Fontebasso AM, Papillon-Cavanagh S, Schwartzentruber J, et al. Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nat Genet 2014;46:462-6. [Crossref] [PubMed]
- Buczkowicz P, Hoeman C, Rakopoulos P, et al. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet 2014;46:451-6. [Crossref] [PubMed]
- Wu G, Diaz AK, Paugh BS, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet 2014;46:444-50. [Crossref] [PubMed]
- Warren KE, Killian K, Suuriniemi M, et al. Genomic aberrations in pediatric diffuse intrinsic pontine gliomas. Neuro Oncol 2012;14:326-32. [Crossref] [PubMed]
- Paugh BS, Broniscer A, Qu C, et al. Genome-wide analyses identify recurrent amplifications of receptor tyrosine kinases and cell-cycle regulatory genes in diffuse intrinsic pontine glioma. J Clin Oncol 2011;29:3999-4006. [Crossref] [PubMed]
- Miyahara H, Yadavilli S, Natsumeda M, et al. The dual mTOR kinase inhibitor TAK228 inhibits tumorigenicity and enhances radiosensitization in diffuse intrinsic pontine glioma. Cancer Lett 2017;400:110-6. [Crossref] [PubMed]
- Becher OJ, Hambardzumyan D, Walker TR, et al. Preclinical evaluation of radiation and perifosine in a genetically and histologically accurate model of brainstem glioma. Cancer Res 2010;70:2548-57. [Crossref] [PubMed]
- Mackay A, Burford A, Carvalho D, et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 2017;32:520-37.e5. [Crossref] [PubMed]
- Hsieh AC, Liu Y, Edlind MP, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 2012;485:55-61. [Crossref] [PubMed]
- Ghobrial IM, Siegel DS, Vij R, et al. TAK-228 (formerly MLN0128), an investigational oral dual TORC1/2 inhibitor: a phase I dose escalation study in patients with relapsed or refractory multiple myeloma, non-Hodgkin lymphoma, or Waldenström's macroglobulinemia. Am J Hematol 2016;91:400-5. [Crossref] [PubMed]
- Hayward RM, Patronas N, Baker EH, et al. Inter-observer variability in the measurement of diffuse intrinsic pontine gliomas. J Neurooncol 2008;90:57-61. [Crossref] [PubMed]
- Hollborn M, Bringmann A, Faude F, et al. Signaling pathways involved in PDGF-evoked cellular responses in human RPE cells. Biochem Biophys Res Commun 2006;344:912-9. [Crossref] [PubMed]
- Zhang H, Bajraszewski N, Wu E, et al. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Invest 2007;117:730-8. [Crossref] [PubMed]
- Song N, Huang Y, Shi H, et al. Overexpression of platelet-derived growth factor-BB increases tumor pericyte content via stromal-derived factor-1α/CXCR4 Axis. Cancer Res 2009;69:6057-64. [Crossref] [PubMed]
- Mittapalli RK, Chung AH, Parrish KE, et al. ABCG2 and ABCB1 limit the efficacy of dasatinib in a PDGF-B–Driven brainstem glioma model. Mol Cancer Ther 2016;15:819-29. [Crossref] [PubMed]
- Shih AH, Dai C, Hu X, et al. Dose-dependent effects of platelet-derived growth factor-B on Glial Tumorigenesis. Cancer Res 2004;64:4783-9. [Crossref] [PubMed]
- Matei D, Kelich S, Cao L, et al. PDGF BB induces VEGF secretion in ovarian cancer. Cancer Biol Ther 2007;6:1951-9. [Crossref] [PubMed]
- Halvorson KG, Barton KL, Schroeder K, et al. A high-throughput in vitro drug screen in a genetically engineered mouse model of diffuse intrinsic pontine glioma identifies BMS-754807 as a Promising therapeutic agent. Plos One 2015;10:e0118926 [Crossref] [PubMed]
- Grasso CS, Tang Y, Truffaux N, et al. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med 2015;21:555-9. [Crossref] [PubMed]
- Nagaraja S, Vitanza NA, Woo PJ, et al. Transcriptional dependencies in diffuse intrinsic pontine glioma. Cancer Cell 2017;31:635-52.e6. [Crossref] [PubMed]
- Hashizume R, Andor N, Ihara Y, et al. Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma. Nat Med 2014;20:1394-6. [Crossref] [PubMed]


