
Association between radiosensitivity and molecular subtypes in patients with early-stage breast cancer and lymph node-negative status
Previous studies have reported that the use of radiotherapy (RT) following breast conserving surgery (BCS) had similar clinical outcomes when compared with that of mastectomy (1,2). The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), which performed a meta-analysis of 10,801 breast cancer patients’ data obtained from 17 randomized trials comparing RT with no RT after BCS, revealed that RT decreased the risk of locoregional and distant recurrences at 10 years, from 35.0% to 19.3%, and subsequently resulted in an increased survival of 3.8% at 15 years. RT also resulted in an absolute recurrence reduction of 15.4% (10 years, no RT vs. RT; 31.0% vs. 15.6%, P<0.00001), as well as an absolute mortality reduction of 3.3% (15 years, no RT vs. RT; 20.5% vs. 17.2%, P=0.005) in patients with lymph node (LN)-negative status (3). When analyzing the variables of clinicopathological features associated with recurrence in LN-negative patients, the authors demonstrated that the 10-year recurrence was approximately 10% after RT even in patients with T1 lesions or low-grade histology (3). These findings indicate that further investigations of potential markers associated with the radio resistance in LN-negative patients with early-stage breast cancer are warranted.
Although the EBCTCG study revealed that most patients with LN-negative early breast cancer benefited from RT owing to the decreased recurrence risk and modality, a substantial proportion of patients, including elderly women with T1 and estrogen receptor (ER)-positive invasive breast cancer who were treated with tamoxifen, did not develop local recurrence in the absence of RT (4). Chesney et al. (5) in an attempt to clarify the question of whether the effect of tamoxifen was equivalent to RT in elderly women with early breast cancer, reviewed four randomized trials that compared the administration of RT plus tamoxifen and tamoxifen alone in 2,387 women aged ≥70 years with T1-T2N0 breast cancer. They showed that RT significantly decreased local recurrence of breast [at 5 years, relative risk (RR), 0.18; 95% CI, 0.10–0.34] and minimally reduced axillary LN recurrence (RR, 0.28; 95% CI, 0.10–0.81) (6-9). However, the distant recurrence and overall survival (OS) were not significantly different between these two groups in these patients’ women (6-9). At 10 years of follow-up, the Toronto/British Columbia trial showed that adjuvant RT resulted in a risk ratio of 0.40 for breast recurrence (95% CI, 0.12–1.25) with 2.9% and 7.5% recurrence being observed from the total number of patients in the RT plus tamoxifen (4 of 135 cases) and tamoxifen alone groups (9 of 120 cases), respectively (8). These findings indicate that RT can be omitted in several elderly women with early-stage breast cancer (T1-T2N0) who received adjuvant endocrine therapy, after evaluating the benefits of RT mediated reduction of recurrence against its side effects.
Based on genes expression profiles and their association with prognosis, breast cancer can be classified into different molecular subtypes (10,11). After using immunohistochemistry and fluorescence in situ hybridization (FISH) techniques to assess the expression patterns of ER, progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), the heterogeneity of breast diseases was categorized as follows: luminal A/B [hormone receptor (HR)+/HER2−, HR+, ER+, and/or PR+], luminal HER2 (HR+/HER2+), HER2 (HR−/HER2+), and triple-negative (HR−/HER2−) (12-15). After examining the Ki-67 index, in addition to ER, PR, and HER2 status, Cheang et al. proposed an immunohistochemical classifier, defining the luminal A subtype as HR+/HER2− with low Ki-67 levels (Ki-67 index <14%), and the luminal B subtype as HR+/HER2− with high Ki-67 levels (Ki-67 index ≥14%) (13,15).
To assess whether RT can be omitted after BCS in specific elderly patients with low-risk early-stage breast cancer, Liu et al. analyzed the ER, PR, HER2, CK5/6, epidermal growth factor receptor, and Ki-67 markers in tissue microarray (TMA) comprising of 501 cases obtained from the prospective Toronto/British Columbia trial (16). In their study, patients were divided into six molecular subtypes according to the expression patterns of the aforementioned six markers as follows: luminal A (n=265), luminal B (n=165), and the high-risk [luminal HER2 (n=22), HER2 enriched (n=13), basal-like (n=30), and triple-negative non-basal (n=6)]. Liu et al. showed that the 10-year ipsilateral breast tumor recurrence (IBTR) rates for luminal A, luminal B, and the high-risk subtypes were 5.2%, 10.5% and 21.3% (P<0.001), respectively (16). Following RT, the IBTR rate for the high-risk subtype was significantly decreased [hazard ratio (HR), 0.13] than both the luminal A (HR, 0.40) and luminal B (HR, 0.51) subtypes (16) (Figure 1).
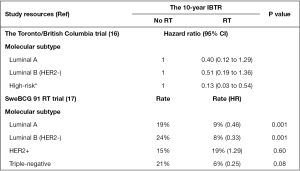
Recently, a 15-year follow-up by the Swedish Breast Cancer Group randomized trial (SweBCG 91 RT) involving 1,187 women with post-BCS T1-2N0M0 breast cancer randomized into whole breast RT or observation (stage II, all received adjuvant systemic therapy) groups revealed patients who received RT following BCS demonstrated lower IBTR (11.5% vs. 23.9%, P<0.001) and better recurrence-free survival (60.4% vs. 51.7%, P=0.0013) than those treated with only BCS (18). However, the OS was not significantly different between the two groups (RT vs. observation groups: 71.1% vs. 68.4%; P=0.68) (18). Furthermore, they also noted that clinicopathological features, such as grade, tumor size, and ER status, could not be used to identify whether patients could be omitted from undergoing RT (18). To further assess whether molecular subtypes of breast cancer were associated with radiosensitivity, Sjöström et al. correlated IBTR and breast cancer death (BCD) with the expression of ER, PR, HER2, and Ki-67 in TMA from 1,003 patients who had been enrolled in the SweBCG 91 RT trial (17). They showed that when compared with the observation group, RT significantly reduced the IBTR within 10 years of follow-up in cases of luminal A subtype (9% vs. 19%, P=0.001), luminal B subtype (9% vs. 19%, P=0.001), and triple-negative subtype (9% vs. 19%, P=0.001); in contrast, the IBTR for the HER2 subtype was not different between the RT (19%) and the observation (15%, P=0.6) groups (Figure 1) (17). Regarding the association between RT and BCD, only triple-negative tumors benefited from RT (HR, 0.35, P=0.06) (17). However, in triple-negative tumor and HER2 subtypes, the number of unbalanced cases and those detected by screening, age, tumor size, menopausal status, and adjuvant treatment with or without RT may influence the effect of RT in decreasing recurrence of breast cancer (17). For example, patients with HER2-positive breast cancer subtypes (45 cases from SweBCG 91 RT trial), had a relatively favorable prognosis despite not being treated with RT (17). The molecular analyses of several patients obtained from the SweBCG 91 RT have demonstrated that luminal A, luminal B, and triple-negative breast tumors are more sensitive to radiation, whereas HER2-positive tumors are relatively radio resistant.
Regarding the benefit of RT on the luminal A subtypes, Sjöström et al. showed that RT reduced the IBTR from 20% to 6% at 10 years in the low-risk group (ER-positive, N0, and age ≥65 years) (17), whereas Liu et al. showed that the 10-year IBTR for RT plus tamoxifen and tamoxifen only in the low-risk group (age >60 years, T1, and grade 1 or 2) was 1.3% and 5%, respectively (16) (Figure 1). The difference in the RT effect on IBTR between the two studies may be explained by the rare administration of tamoxifen (around 5% of the patients received tamoxifen) in the study by Sjöström et al. (17) when compared to all the patients receiving tamoxifen in Liu et al. (16) study, since adjuvant endocrine therapy may also facilitate decreased risk of recurrence (6-9,19). In addition, the decreased number of events of IBTR in clinical low-risk luminal A subtypes may also have attributed to decreasing the benefit from RT in the study by Liu et al. (16). These findings indicate that adjuvant endocrine treatment, such as tamoxifen and aromatase inhibitors, may be considered as an alternative to RT in elderly women with clinical low-risk luminal A-like breast tumors. On the other hand, RT should be considered as an alternative therapy to decrease the risk of breast cancer recurrence in elderly women whose clinical low-risk luminal A subtype breast tumors are either unsuitable for or unable to tolerate endocrine therapy.
The association between radiosensitivity and luminal A-like, HER2-positive, and triple-negative breast tumors have been reported in few retrospective studies (14,20,21). For example, Kyndi et al. investigated the correlation of the expression of ER, PR, and HER2 with the response to post-mastectomy RT (PMRT) in the combined Danish 82b and 82c clinical trials, and disclosed that PMRT significantly reduced the 10-year locoregional recurrence (LRR) in HR+/HER2− breast tumors (PMRT vs. no RT, 3% vs. 32%, P<0.001), HR+/HER2+ breast tumors (PMRT vs. no RT, 3% vs. 48%, P<0.005), and in triple-negative breast tumors (PMRT vs. no RT, 15% vs. 32%, P=0.001), but not in HR−/HER2+ breast tumors (PMRT vs. no RT, 21% vs. 33%, P=0.2) (21). However, the interpretation of radio resistance of HR-/HER2+ breast tumors from the Danish 82b and 82c clinical trials should be cautiously established (21), since most of these patients had N1 and not all patients received anti-HER2 treatment, such as trastuzumab. The recent update of the HERA (HERceptin Adjuvant) trial, reported that the 10-year disease-free survival (DFS) in early stage breast cancer patients with HER2-positive (T1-T3, N0-2, and 50% positivity for ER or PR) was 63% and 69% for the observation and 1-year trastuzumab treatment (22). Although the post-BCS patients who entered the HERA trial (22) were all treated with adjuvant RT, it was noted that their 10-year DFS was relatively better than that of the HER2-positive patients who received RT in the study by Sjöström et al. (17). Another retrospective trial conducted on women with T1-T2N0 and HER2-positive breast cancer who received BCS and RT, showed that the 3-year LRR-free survival rate in the trastuzumab and no-trastuzumab cohort were 99% and 90%, respectively (23). Further investigation of the association between radio resistance in women with T1-T2N0 and HER2-positive breast cancer subtypes who received trastuzumab or no-trastuzumab treatment is warranted.
In a retrospective study assessing the relationship between radiosensitivity and triple-negative breast tumors, Abdulkarim et al. revealed that women with T1-2N0 triple-negative breast tumors treated with modified radical mastectomy without RT had a worse 5-year LRR-free survival than those treated with BCS and RT (90% vs. 96%, P=0.027) (24). We also found that in women with T1-2N1 breast cancer who received modern systemic chemotherapy without PMRT, patients with triple-negative breast tumors were significantly associated with a higher 5-year LRR rate than those without triple-negative breast tumors (10.6% vs. 4.2%, P=0.05) (25). These findings indicate that, even after modern systemic chemotherapy and endocrine therapy, patients with early-stage triple-negative breast cancer may have high LRR risk; therefore, this subgroup of patients may need aggressive locoregional treatment, such as locoregional RT, following BCS or mastectomy. However, owing to the limitations of previous prospective or retrospective studies that have investigated the association between triple-negative breast tumors and LRR in patients with early-stage breast cancer (T1-2N0) who received mastectomy alone or BCS plus RT, further clarifications of whether patients with triple-negative breast cancer can benefit from PMRT and an additional regional lymph nodal RT following BCS are warranted.
Although the results obtained by Liu et al. showed that the luminal B breast cancer subtype was not associated with radiosensitivity (16), the use of RT was closely associated with decreased recurrence in luminal B breast cancer subtype in the study established by Sjöström et al. (17). Immunohistochemical staining of ER, PR, HER2, and Ki-67 cannot represent the biologic behavior of luminal B breast cancer subtype; thus, further studies using alternative robust assessment techniques, such as PAM50 (26), to correlate LRR and DFS in a large cohort of women with T1-2N0 breast cancer are warranted.
Based on the results from the studies established by Liu et al. (16) and Sjöström et al. (17), as well as from retrospective studies, we speculated that among women with T1-2N0 breast cancer (1), the luminal A breast cancer subtype seems to be associated with radiosensitivity in high-risk patients (age ≤65 years, grade 2 to 3) and those not treated with endocrine therapy (2), and triple-negative breast cancer seems to be highly benefited from RT. However, further studies investigating the association between luminal A and triple-negative breast cancer subtypes and radiosensitivity are warranted. Indeed, there are several ongoing prospective trials assessing whether RT can be omitted in the clinical low-risk luminal A breast cancer subtype to prevent effects on OS. For example, a prospective, single-arm clinical trial by the Ontario Clinical Oncology Group, is assessing whether the local recurrence is less than 5% in women with 55 years of age or older, with pT1N0, grade 1 to 2, and luminal A breast cancer subtype who received BCS and endocrine therapy without RT (Clinicaltrials.gov identifier NCT01791829). To avoid potential under-treatment in women with T1-2N0 and triple-negative breast cancer, a prospective clinical trial is needed to assess the effect of regional lymph nodal irradiation plus whole breast RT in post-BCS patients or RT in post-mastectomy patients.
Acknowledgments
Funding: This study was supported by research grants MOST 104-2314-B-002-152-MY3 from the Ministry of Science and Technology, Taiwan.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor San-Gang Wu (Department of Radiation Oncology, Xiamen Cancer Center, the First Affiliated Hospital of Xiamen University, Xiamen, China)
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.11.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [Crossref] [PubMed]
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707-16. [Crossref] [PubMed]
- Tinterri C, Gatzemeier W, Costa A, et al. Breast-conservative surgery with and without radiotherapy in patients aged 55-75 years with earlystage breast cancer: A prospective, randomized, multicenter trial analysis after 108 months of median follow-up. Ann Surg Oncol 2014;21:408-15. [Crossref] [PubMed]
- Chesney TR, Yin JX, Rajaee N, et al. Tamoxifen with radiotherapy compared with Tamoxifen alone in elderly women with early-stage breast cancer treated with breast conserving surgery: A systematic review and meta-analysis. Radiother Oncol 2017;123:1-9. [Crossref] [PubMed]
- Kunkler IH, Williams LJ, Jack WJ, et al. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol 2015;16:266-73. [Crossref] [PubMed]
- Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med 2004;351:971-7. [Crossref] [PubMed]
- Fyles AW, McCready DR, Manchul LA, et al. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med 2004;351:963-70. [Crossref] [PubMed]
- Fisher B, Bryant J, Dignam JJ, et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol 2002;20:4141-9. [Crossref] [PubMed]
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. [Crossref] [PubMed]
- Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869-74. [Crossref] [PubMed]
- Cheang MC, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res 2008;14:1368-76. [Crossref] [PubMed]
- Cheang MC, Chia SK, Voduc D, et al. Ki67 Index, HER2 status, and prognosis of patients with luminal B breast Cancer. J Natl Cancer Inst 2009;101:736-50. [Crossref] [PubMed]
- Voduc KD, Cheang MC, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol 2010;28:1684-91. [Crossref] [PubMed]
- Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol 2013;24:2206-23. [Crossref] [PubMed]
- Liu FF, Shi W, Done SJ, et al. Identification of a low-risk luminal a breast cancer cohort that may not benefit from breast radiotherapy. J Clin Oncol 2015;33:2035-40. [Crossref] [PubMed]
- Sjöström M, Lundstedt D, Hartman L, et al. Response to radiotherapy after breast-conserving surgery in different breast cancer subtypes in the Swedish Breast Cancer Group 91 radiotherapy randomized clinical trial. J Clin Oncol 2017;35:3222-9. [Crossref] [PubMed]
- Killander F, Karlsson P, Anderson H, et al. No breast cancer subgroup can be spared postoperative radiotherapy after breast-conserving surgery. Fifteen year results from the Swedish Breast Cancer Group randomised trial, SweBCG 91 RT. Eur J Cancer 2016;67:57-65. [Crossref] [PubMed]
- Winzer KJ, Sauerbrei W, Braun M, et al. Radiation therapy and tamoxifen after breast-conserving surgery: Updated results of a 2 x 2 randomised clinical trial in patients with low risk of recurrence. Eur J Cancer 2010;46:95-101. [Crossref] [PubMed]
- Dominici LS, Mittendorf EA, Wang X, et al. Implications of constructed biologic subtype and its relationship to locoregional recurrence following mastectomy. Breast Cancer Res 2012;14:R82. [Crossref] [PubMed]
- Kyndi M, Sørensen FB, Knudsen H, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: The Danish Breast Cancer Cooperative Group. J Clin Oncol 2008;26:1419-26. [Crossref] [PubMed]
- Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin adjuvant (HERA) trial. Lancet 2017;389:1195-205. [Crossref] [PubMed]
- Kiess AP, McArthur HL, Mahoney K, et al. Adjuvant trastuzumab reduces locoregional recurrence in women who receive breast-conservation therapy for lymph node-negative, human epidermal growth factor receptor 2-positive breast cancer. Cancer 2012;118:1982-8. [Crossref] [PubMed]
- Abdulkarim BS, Cuartero J, Hanson J, et al. Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol 2011;29:2852-8. [Crossref] [PubMed]
- Lai SF, Chen YH, Kuo WH, et al. Locoregional recurrence risk for postmastectomy breast cancer patients with T1-2 and one to three positive lymph nodes receiving modern systemic treatment without radiotherapy. Ann Surg Oncol 2016;23:3860-9. [Crossref] [PubMed]
- Chia SK, Bramwell VH, Tu D, et al. A 50-gene intrinsic subtype classifier for prognosis and prediction of benefit from adjuvant tamoxifen. Clin Cancer Res 2012;18:4465-72. [Crossref] [PubMed]


