Innovative surgical treatment of peripheral lymphedema after breast cancer surgery
Introduction
The main focus of medical research and development has been directed mainly to the immunologic function of lymphatic system because infectious disease and cancer are the most common diseases affecting human body worldwide.
The treatment of breast cancer usually requires ablative breast surgery and node surgery which may vary from sentinel node biopsy to axillary clearance.
Being Axillary nodes responsible for the lymphatic drainage of both breast and ipsilateral upper limb, oncologic treatments (surgery and adjuvant treatments) directed to those structures may jeopardize the lymphatic drainage of the ipsilateral upper limb and finally lead to the development of upper limb lymphedema.
Extremity lymphedema is a chronic, progressive and debilitating disease characterized by imbalance of lymphatic flow dynamics which manifest with the following clinical picture: different degrees and extension of limb swelling, sense of heaviness, neuropathic pain, limited range of motion in advanced cases, higher risk of developing cellulitis and lymphangitis which sometimes may be complicated by septic shock, and rare cases of malignant transformation into lymphangiosarcoma (1) (Figure 1).
Lymphedema is not a rare disease, affecting over 140 million patients worldwide (1). With advances in cancer early diagnosis and improvement in cancer treatment, the number of cancer survivors is rapidly growing and it is expected to continue with this trend. As consequence, the number of patients affected by lymphedema is expected to increase in the next years/decades making this disease one of the most common debilitating outcome affecting quality of life of cancer survivors.
In advanced countries, 98% of upper extremity lymphedema (UEL) are secondary to breast cancer treatments. The risk factors for its development are: axillary clearance although there are few reports of UEL after sentinel node biopsy, number of nodes removed, cancer invasiveness, radiation therapy either toward axilla and breast, body mass index (BMI) over 30, genetic predisposition (mutations of VEGFR2, VEGFR3, RORC and SNPs in the receptor genes) (1).
It has been reported that UEL can be registered in 5% to 41% of breast cancer patients and in Italy it has been reported an incidence of around 14,000 new cases/year. 70% of the patients shows disease onset within the first 2 years after cancer treatments.
Nevertheless this disease is quite common, it has been usually neglected and underestimated due to the scarce knowledge about the lymphatic system and the unavailability of effective treatments. As consequence, breast cancer patients are usually not well instructed about the risk of this disease and once UEL is diagnosed they are usually not follow-up by doctors but send for consultation to physical therapist only.
The inadequate counselling about this disease lead to its underestimation even in women which are affected. The results are that patients are referred to lymphedema specialists usually when the disease is already in advanced stage, limiting treatment rate of success.
UEL has a great impact of QoL of cancer survivors as suggested by different studies. Moreover, it has been demonstrated that it has also a strong socioeconomical impact either for the single patient and for society economic burden. This is very likely related to the frequent absence of an organized network dedicated to this disease which is usually not approached systematically (2-5).
Since a decade, the surgical treatment of lymphedema has expanded in breath and scope with the introduction of microsurgical physiological procedures such as lymphaticovenous anastomosis (LVA) and lymph node flap (LNF) transfer.
Both procedures have been demonstrated to be effective in the treatment of not fibrotic lymphedema. However, outcomes are still very operator dependent, thus level of evidence remains low and standardized surgical protocol are lacking.
The aim of this paper is to provide the status of art of diagnosis and microsurgical treatment of UEL according to the experience of three centers, which provide those treatment as standard-of-care.
Diagnosis
Preoperative imaging becomes mandatory in order to understand the functional status of lymphatic collectors and the equity of lymphedema. These informations are helpful in choosing the indication for the physiologic procedure to be performed as well as may help in predicting the outcome.
Lymphatic network imaging has been underdeveloped as lymphedema has usually being neglected as pathology of medical interest and its treatment has been delegated to physiotherapist. However, physiotherapy may offer symptomatic and transient amelioration but it cannot be curative as it does not interfere with the spiral of decline of the disease.
In the last decade, many advances have been made in lymphatic network imaging and nowadays the lymphatic microsurgeon can take advantage on the preoperative imaging.
Based on the concept behind each diagnostic method, today we can group the lymphatic imaging in the following three categories:
(I) Direct method (intraluminal)
Direct method refers to the technique of visualization of lymphatic network by intra-lymphatic injection of contrast substance. However, differently from peripheral vein cannulation, peripheral lymphatic cannulation is a more technically demanding procedure. The first reported imaging method for lymphatic network visualization has been the oil-contrast lymphography. This method has been used in the assessment of nodal metastases in lymphoma and other cancers before the introduction of CT and MRI which have completely replaced it. It has been reported also to study lymphatic network in patients suffering lymphedema.
Oil-contrast lymphography
This procedure has been abandoned for several reasons. It is an invasive procedure that place a scar and expose patients to high dosage of radiation, it is time consuming and highly expensive. The oil contrast medium has also been demonstrated to be toxic on lymphatic endothelium (oil-injured lympho-angiopathy). Moreover, life-threatening complications have been reported with oil-contrast lymphography such as pulmonary embolism and respiratory distress syndrome. Finally, major indications for which lymphography had been developed are now largely covered by CT and MRI. According to Campisi and Boccardo, oil-contrast lymphography should be limited for patients with chylous reflux syndrome, where more precise visualization of retroperitoneal collectors may be required (6,7).
(II) Physiologic methods
Nowadays, physiologic methods represent the mainstay of lymphatic network anatomy imaging and functionality evaluation.
The concept behind these methods is to inject the contrast in the interstitial space so that the tracer can be picked up by lymphatic capillaries and be drained by the lymphatic network. There are different physiologic methods available, each with its own advantages and limits. These methods can be combined in the surgical planning of lymphatic microsurgery.
Lymphoscintigraphy
Lymphoscintigraphy with radioisotope-labelled colloids for the clinical study of lymphatic network has been introduced in the 60s. However, it has been officially recognized as valid imaging method in the diagnosis and evaluation of lymphedema only in 2004 by the International Society of Lymphology (ISL) Consensus Document as it was considered still experimental.
The administration route and technical methodologies are also very important in defining the subtype of lymphoscintigraphy performed and consequently the timing and informations that each technique can provide.
The standard lymphoscintigraphy method that is usually performed to study the lymphatic network is represented by the rest subcutaneously lymphoscintigraphy.
(i) Subcutaneous rest lymphoscintigraphy (SubQ-RL)
This method is not yet standardized and may vary among different centres for choice of injection site, for using dynamic rather than static acquisition and for choice of acquisition time.
However, this method is usually performed by injecting 0.1 to 0.3 cc of radio-colloid diluted solution in the 1st interdigital space of foot or hands with particular care to avoid any intravascular injection.
After injection, two imaging are usually acquired: early and late scans. Early scans may take up to 30–60 mins whereas late scans are acquired from 3 to 6 hours from injection. In this timing, patient is asked to avoid strenuous activity in order to obtain information at rest.
The evaluation of the findings is made based on clearance rate of the colloid from injection site, percentage of uptake by loco-regional lymph node, delay in lymphatic drainage, asymmetric or absence visualization of loco-regional nodes, presence of collateral collectors and dermal backflow (DB). Moreover, transport index (TI) is a quantitative method used to classify the severity of lymphatic drainage impairment as proposed by Weiss and Baumeister (8).
Although SubQ-RL is the most diffused method, it shows several limitations especially in the preoperative planning of physiologic microsurgical treatment for lymphedema.
Rest-stress lymphoscintigraphy (RSL) is time-consuming exam, with poor image resolution due to high background activity from injection sites, blood, liver and bladder which makes very difficult also a univocal interpretation of result and TI evaluation.
In our experience with RSL, we found this method not useful for a detailed quantitative and qualitative analysis of lymphedema acuity and of residual collector function, especially for advanced lymphedema stage. We believe that both parameters are very important either in choosing the physiologic treatment to propose to patient (LVA vs. LNF) as well as in planning the surgery.
We found that Intradermal RSL according to Tartaglione (9,10) is able to overcome limitations of standard RSL.
(ii) Intradermal RSL (IDI-RSL)
In Italy, IDI-RSL represents our preferred method to preoperatively study patient with lymphedema. This method gives us clear quantitative and qualitative data for lymphedema staging and surgical planning. As for surgical protocol, we combine data from IDI-RSL with that coming from indocyanine green (ICG)-lymphography in order to plan the microsurgical treatment. IDI-RSl has been introduced by Tartaglione in 2010 (9,10). Briefly, diluted 99mTc-nanocolloids in neutral pH solution are intradermally injected into first interdigital space of hand or feet. The acquisition protocol takes less than 1 hour only and it is differentiated in rest scan (within the first 10 minutes), stress scan (after 2 minutes of exercise) and late scan (30 to 60 minutes after injection). Besides TAT, lymphedematous limb shows different IDI-RSL patterns which correlate which lymphedema acuity.
We believe that IDI-RSL is methodologically superior to SubQ-RL, both in lymphedema diagnosis and in preoperative planning (Figure 2).
Fluorescence ICG-lymphography
ICG is useful for near-infrared fluorescent lymphography, which allows the clearest visualization of superficial lymph flows in real-time without radiation exposure (11-14). ICG lymphography has been widely applied in lymphedema management; diagnosis and pathophysiological severity staging of arm, leg, facial, and genital lymphedema, and intraoperative navigation for lymphatic surgeries (13,14). ICG lymphography is performed as follows: 0.1 mL of 0.25% ICG is injected intra- or subcutaneously at the second web space of the hand and at the wrist just ulnar to the palmaris longus tendon (15). Fluorescent images are obtained using a near-infrared camera immediately after ICG injection (transient phase) and 2–72 hours after injection (plateau phase); dual-time or dynamic ICG lymphography. ICG lymphography allows lymphedema diagnosis/evaluation, severity staging, and intraoperative navigation by one ICG injection (15).
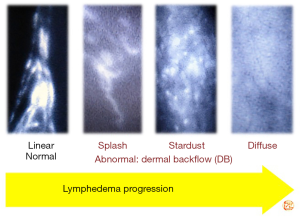
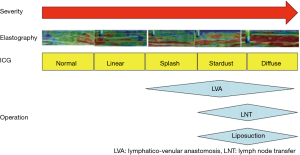
ICG lymphography findings
ICG lymphography findings are largely classified into two patterns: normal Linear pattern, and abnormal DB pattern (14,15). DB pattern is subdivided into 3 patterns: mild DB, Splash pattern; moderate DB, Stardust pattern; severe DB, Diffuse pattern (14,15). With lymphedema progression, ICG lymphography findings change from Linear, to Splash, to Stardust, and to Diffuse pattern (Figure 3). Under normal conditions, flows of collecting lymphatic vessels are shown as Linear pattern on ICG lymphography. After lymph flow obstruction, collecting lymphatic vessels become dilated, and lymphatic valvular insufficiency takes place, leading to retrograde lymph flows. Due to retrograde lymph flows, more superficial lymphatics such as lymphatic pre-collectors and capillaries also becomes dilated; these dilated superficial lymphatics, working as collateral lymph pathways, are seen as Splash pattern on ICG lymphography. When these collaterals fail to compensate lymph overload, lymph cannot be drained via lymphatics and retains in the interstitial space; congested lymph of vertical retrograde lymph flows (to the skin) is shown as Stardust pattern. Finally, the most superficial lymphatic capillaries become totally dilated because of no lymphatic collectors’ lymph flow, which is shown as Diffuse pattern.
Pathophysiological severity staging (DB stage)
In DB stage 0, only Linear pattern is seen and DB pattern not. In DB stage I, Splash pattern is seen around the axilla. In DB stages II–V, Stardust or Diffuse pattern is seen. Stardust/Diffuse pattern is seen in 1 region in DB stage II, in 2 regions in DB stage III, in 3 regions in DB stage IV, and whole area of the upper extremity (Linear pattern is not seen) in DB stage V; the upper extremity is divided into 3 regions including the upper arm, the forearm, and the hand (14,15).
Since no patients can be controlled under conservative treatment alone, lymphatic surgery is recommended, especially when edema is clinical evident.
Single-photon emission computed tomography (SPECT)
The diagnostic and therapeutic strategies against lymphedema remain unestablished in the clinical setting of lymphedema, although investigators have struggled to reveal the actual mechanism of lymphodynamic disorders.
Lymphoscintigraphy using 99mTc-human serum albumin diethylenetriamine pentaacetic acid (99mTc-HSAD) is commonly adopted to evaluate the lymphodynamic condition in lymphedema patients, and its type categorization using this modality has already been established (16-19). However, as 99mTc-HSAD promptly distributes to entire vascular systems due to its small particle size (2–3 nm), discriminating lymphatic vessels from veins may prove to be difficult.
Compared to this, 99mTc-phytate has a larger particle size (200–1,000 nm) and is trapped in axillary lymph nodes (5). Such characteristics of this tracer might facilitate further comprehension of lymphodynamic conditions, including main lymphatic vessels’ flow and lymphovenous shunt.
Recently, SPECT-computed tomography (SPECT-CT) combined systems have been introduced for clinical use. We believe that 99mTc-phytate lymphoscintigraphy, integrated with SPECT-CT fusion image, allows us to interpret the detailed lymphodynamic conditions by combining functional and morphological information and helps to establish a new clinical staging system for upper-limb lymphedema (20,21). This modality may also promote the comprehension of surgical therapeutic efficacy.
Lymphoscintigraphic Imaging with SPECT-CT
Tc-99m-phytate 185 MBq in a total volume of 1 mL was subcutaneously injected into the second interdigital spaces on both sides of the hand by using a 30-gauge needle. The interval between the 2 injections was less than 30 seconds. Injection sites were massaged for 3 minutes to facilitate tracer transport into lymphatic systems. Acquisitions were performed using a SPECT-CT combined system equipped with a dual-headed gamma camera. Anterior and posterior planar images from neck to hand were acquired, starting at 2-time points, 15 and 90 minutes after the injection. The acquisition from each time point proceeded over 10 minutes. SPECT-CT images from hand to chest area were acquired over 30 minutes, starting at 25 minutes after the injection, and a total of 30 projection images were obtained over an orbit of 360 degrees in 6-degree increments at a rate of 30 seconds per projection. The image matrix size was 128, the pixel size was 4.8 mm, and a low energy high-resolution collimator was used. Images were acquired on the 140 keV photopeak, with a 20% symmetrical energy window.
In both early and delayed lymphoscintigraphic images with SPECT-CT, the lymphodynamic findings, including lymphatic vessel accumulation, axillary lymph node accumulation, and dermal backflow, were interpreted with the consensus of experienced nuclear radiologists.
99mTc-phytate lymphoscintigraphy with SPECT-CT can provide both functional and morphological information simultaneously in patients with upper-limb lymphedema (Figures 4,5).
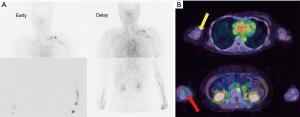
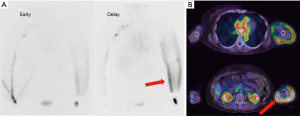
Using this modality, SPECT-CT may accurately reflect their lymphodynamic conditions. Furthermore, the surgical therapeutic efficacy could be estimated quantitatively by comparing the pre- and postoperative findings.
Lymph-MRI
MRI lymphangiography
Although lymphoscintigraphy, especially if performed with IDI-RS method, allows to obtain very valuable quantitative data, the quality of image resolution remains poor and it is not possible to have an anatomical detailed map of the lymphatic network.
In this perspective, high resolution imaging methods such as CT and MRI have been explored in the last decade. MRI has been the preferred method investigated as it does not expose the patient to ionizing radiation, it provides high resolution three-dimensional imaging of the superficial and deep lymphatic network as well as functional information, it allows to map percutaneously the site of interest and it can be used to evaluate other informations such as subcutaneous fat thickness, amount of lymph stasis and limb volumetry.
The reported contrast medium used to perform MR lymphangiography have been gadolinium-labeled diethylenetriaminepentaacetic acid (Gd-DTPA), gadolinium dendrimers or liposomes and iron oxide particles. Although few reports are available with MR lymphangiography in the diagnosis of lymphedema, this methodology seems promising.
MRI lymphangiography may be very useful to depict lymphatic collector degeneration patterns as well as may be very useful in the assessment of lymphatic collector and/or lymph node malformation in patients with primary lymphedema (22-24).
At the best of our knowledge, none have yet explored this method as preoperative tool for physiologic microsurgery for the treatment of lymphedema. As this method is rapidly evolving in the diagnosis of lymphedema, we believe that this imaging tool may be very promising in surgical planning for the treatment of lymphedema.
(III) Indirect methods-ultrasound
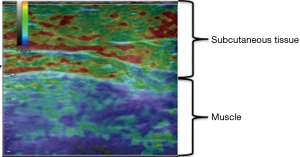
Ultrasound elastography
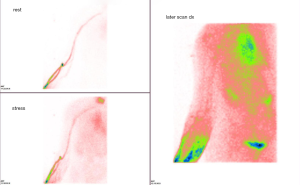
Nowadays, elastography which is performed by ultrasonography could be a useful alternative evaluation for lymphedema severity when ICG lymphography is not available. Elastography is a relatively new ultrasonographic technique to evaluate tissue elasticity, which visualize fluid retention as a red region in lymphedema patients (25-27). Ultrasound elastography is an objective quantitative measurement for the diagnosis and evaluation of edema (Figure 6). It was reported that elastography has the correlation to ICG lymphology (26). As ICG pattern progressed, red region area was likely to increase. Elastography can determine whether an arm is physiologically normal, or lymphedematous to a certain degree (moderate to severe). Elastography is useful to indicate of surgery for lymphedema patients. Since elastography is performed by ultrasonography which is available in most institutions, elastography could be a useful alternative evaluation for lymphedema severity (Figure 7).
Ultrasonography detection of lymphatic vessel
For detection of lymphatic vessels in the site where dermal backflow pattern was shown in ICG lymphography or ICG is not able to be used, the ultrasound could be a substitute for ICG lymphography. It was reported that lymphatic vessels were identified as intermittent homogeneous, hypoechoic and specular misshapen images using ultrasonography (27).
The most important things for detection of lymphatic vessels are distinguishment from blood vessels and nerves. Each has each characteristic in shape, echogenic texture and color Doppler mode. It is desirable to use a linear probe in color Doppler mode at a frequency of more than 15 MHz for precise distinguishment.
ICG lymphography has a depth limit, detecting lymphatic vessels only within 1.5–2 cm of the body surface. Some lymphatic vessels in the upper arm region exist 2 to 3 cm from the skin. Thus, detection of lymphatic vessel in the upper arm is difficult with ICG lymphography. Lymphatic vessels in the upper arm sometimes reside in rich fatty tissue in the deep layer, making their detection consistently challenging for surgeon. Because LVA incisions in the upper arm are placed mainly according to the experience of surgeons, non-detection of lymphatic vessels in some incisions is a common occurrence. Preoperative ultrasound detection of lymphatic vessels resolves this uncertainty, tell surgeon exact location of lymphatic vessels even in the deep layer and reside in rich fatty tissue.
Finding venules appropriate for anastomosis to the detected lymphatic vessels intraoperatively is also difficult and demanding. Surgeons face a situation in which there is a suitable lymphatic vessel but no suitable venule for lymphaticovenular anastomosis frequently, especially in the forearm region. In such cases, surgeons attempt to extend the incision to find a suitable vein, and this leaves a long scar with no contribution to the therapeutic outcome. Ultrasound can detect not only lymphatic vessels but also venules. Surgeons can select the venule which has suitable size for the diameter of lymphatic vessels easily and know also the location of venules. Surgeons can select also the venule which has less backflow using push and release technique in ultrasound color Doppler mode for prevention of venous reflux at the lymphaticovenular shunt preoperatively. These advantages of preoperative ultrasound detection technique reduce required time for dissecting vessels which use for LVA, and also may increase the postoperative reduction change rate of upper limbs (28).
The presence of large lymphatic vessels with abundant lymph flow is an important factor determining the therapeutic effect of LVA in patients with UEL. With using ultrasound, surgeons can detect and select the lymphatic vessels which has expanded lumen using ultrasound preoperatively. Lymphatic vessels which has expanded lumen in ultrasound express the lymphatic vessels which has still valve function and high-flow. Preoperative ultrasound detection technique of lymphatic vessels has a therapeutic advantage that is independent of lymphatic diameter or lymphatic flow.
Ultrasonography has a higher possibility of usefulness for diagnosis and preoperative planning of lymphedema in most institutions. Ultrasonography can detect lymphatic vessels in dermal backflow pattern of ICG lymphography and where ICG lymphography hardly visualizes lymphatic vessels. ICG lymphography can detect whole lymphatic flows of lower limbs and evaluate and diagnose lymphedema. Taking each characteristic into consideration, detecting of lymphatic vessels using ultrasonography technique would complement ICG lymphography technique for lymphedema patients.
Treatment
In the last decade, new microsurgical and supermicrosurgical approaches have been reported for the treatment of UEL with promising clinical outcomes. Those procedures are also called physiologic methods as they differentiate from previous methods which very directed to reduce volume (debulking procedures) rather than treating lymph stasis.
Debulking procedures such as partial soft tissue resection and whole soft tissue resection such as the Charles procedures are nowadays performed only for very advanced, fibrotic not pitting edema. Charles procedure can be disfiguring and may be complicated by subsequent chronic wounds and infections.
Liposuction is a debulking procedure which in some centers is offered as standard of care and in centers where physiologic treatment is offered, liposuction represents an ancillary procedure with the aim of treat excess adipose tissue which has growth in the lymphedematous limb.
The two main physiologic surgeries which are offered nowadays in tertiary care centers are: LVA and lymphatic tissue/LNF transfer (6,29,30).
Although not the aim of this paper, there is an increasing trend in performing prophylactic LVA during axillary dissection in order to prevent development of lymphedema.
LVA
Lymphaticovenular anastomosis is a physiologic procedure in which lymphatic collectors are bypassed to a recipient venule in order to diverge the lymph flow directly into the venous system, thus respecting physiologic lymphatic drainage.
Lymphaticovenular anastomosis can be performed by using different techniques which does not only differ technically but also on the target lymphatic channels to bypass.
Microsurgical single site multiple LVA described by Campisi aim to bypass lymph flow into the venous system, by telescoping multiple, undissected lymphatic channel into a major recipient venule usually at the level of limb radix (upper third of the arm for UEL patients) (31).
Supermicrosurgical LVA described by Koshima is a different surgical procedure in which a single suprafascial lymphatic channel is dissected and anastomosed to a recipient venule. In this procedure high magnification operating microscope, dedicated supermicrosurgical instruments and advanced microsurgical skills are needed in order to be able to perform anastomosis between vessel with a caliber inferior to 0.8 mm (32).
Differently to Campisi technique, supermicrosurgical LVA are performed through multiple incision along the affected limb, can be customized based on single patient edema features.
Moreover, the main difference between those techniques is related to pathophysiology of lymphedema. In fact, it has been showed by basic science study that lymphatic collectors of a lymphedematous limb undergo progressive degenerative phenomenon and, especially for cancer-related lymphedema, the degeneration processes affect first major central lymphatic channels whereas peripheral collectors are affected later. For this reason, bypassing lymphatic channels which does not yet show degeneration will influence positively the outcomes as also demonstrated by different publications.
Recently, an animal study comparing microsurgical and supermicrosurgical LVA showed superiority of the supermicrosurgical technique (33).
Supermicrosurgical LVA can be also performed to deep lymphatic channel when superficial lymphatic channels show advanced degeneration. Moreover, efferent node lymphatic channel may also be bypassed.
This procedure is considered minimal invasive and can also be performed under local anaesthesia when patient compliance allows it.
LVA is an effective surgical procedure for the treatment of peripheral lymphedema with a reported efficacy in limb volume reduction from 20 to 70%, a reduced incidence of cellulitis and lymphangitis as well as an improved QoL (Figures 8,9) [(34,35) and unpublished personal data].
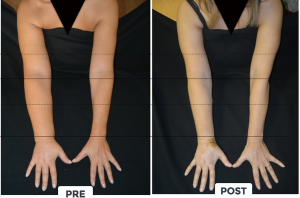
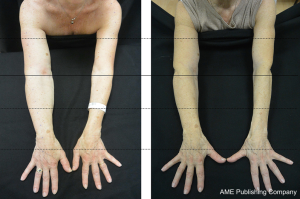
Although it has been described as a procedure with low complication rate, different expert opinions reported a risk of around 5% of patients with edema worsening after LVA, when not correctly planned and performed.
Preoperative LVA planning is based on ICG findings, ultrasound findings (both for locating favourable venules and understanding the status of lymphatic channels), IDI lymphoscintigraphy findings and sometimes SPECT findings.
Flaps
The other physiologic procedure is represented by LNF. This procedure is usually offered to UEL patients when there is an evidence of absent lymphatic channel which may not justify LVA or to patients refractory to LVA. However, UEL patients are usually referred to plastic surgeons for delayed autologous reconstruction after implant reconstruction failure.
This is especially true in radiated patients or in cases where the implant reconstruction failed for other reasons such as capsule contracture, scarring, skin thinning (36,37).
Described donor site for LNF harvest are: submental, supraclavicular, lateral thoracic, groin and recently intraabdominal flaps (38-40). Patients with UEL and indication for delayed breast reconstruction with abdominal flaps may take advantage of single donor-site for both breast reconstruction and lymphedema restoration, such as the deep inferior epigastric artery perforator (DIEP) combined with groin LNF (41).
Groin LNF can be harvested as single flap or can be combined with abdominal flaps, when a one-stage delayed autologous breast reconstruction and lymphedema restoration is performed.
Single stage total breast reconstruction—combined DIEP and groin LNF
The DIEP flap represents today the gold-standard of autologous breast reconstruction as it can be employed in many patients (except very thin patients without abdominal donor site or patients with previous abdominal soft tissue surgery such as abdominoplasty or liposuction) (36). This procedure allows a very pleasant autologous breast reconstruction with minimal morbidity to the donor site and usually an improved cosmesis of the donor-site. We believe that when performing a delayed autologous breast reconstruction, UEL should be always ruled out and if present should be addressed in the same surgery in order to provide a “total” breast reconstruction (including not only the reconstruction of the breast mound but also the restoration of UEL).
In fact, one of the main issues in UEL is axillary vein scar compression due to axillary clearance and radiation therapy. Axillary vein scar release may further improve edema by reducing venous pressure of the affected limb as well as favouring a more successful simultaneous or delayed LVA as recipient venules may show lower intraluminal pressure.
To reduce the risk of iatrogenic lymphedema of the lower extremity, a reverse mapping is mandatory to locate “hot” nodes which drains the lower limb in order to not include them in the LNF flap. We perform reverse mapping as described by Dayan (42). We do not perform it with ICG because ICG will be used intraoperatively to assess flap viability. Three injection points of 0.1 cc of patent blue are performed on the lower abdomen to trace intraoperatively the lymphatic channels which guide the surgeon during the dissection.
Once harvested, perfusion is checked intraoperatively with ICG and LNF is revascularized indirectly (using abdominal perforators when LNF is along zone 1 of perfusion with or without additional venous discharge in the axilla) or directly by anastomosing its pedicle (superficial circumflex iliac artery and vein or superficial epigastric artery and vein) to branch of thoracodorsal vessels (which are always preserved for any back up reconstruction) or to circumflex scapular vessels (Figures 10,11).
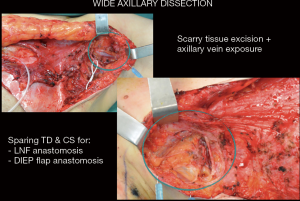
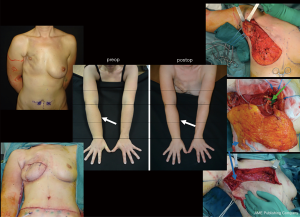
Groin LNF are inserted with the aim to place them in direct contact to the released axillary vein into a pocket with extend to the radix of the upper extremity.
Supermicrosurgical groin LNF
To achieve minimization of donor site morbidities, concepts of supermicrosurgery, true perforator flap, and ICG lymphography-navigated surgery are introduced into LNF surgery (43). Dominant donor site lymph nodes, which should be preserved during flap harvest, are visualized with ICG lymphography reverse mapping. After identification of the donor lymph nodes, the other lymph nodes are selectively dissected with supermicrosurgical techniques as in true perforator flap dissection; “safe” lymph nodes based on tiny nutrient vessels (0.5 mm vessels) are supermicrosurgically harvested and revascularized using recipient limb perforators (Figure 12). Unlike conventional LNF, this supermicrosurgical LNF may allow further preservation of donor lymph flows with significantly less risk of donor site lymphedema and moreover may favour a complete lymph flow reconstruction by anastomosing the efferent lymphatic of the flap to a recipient venule by using supermicrosurgical technique (44).
In conclusion, microsurgical physiologic procedures are gaining widespread popularity, as they are able to improve lymphedema. LVA should be preferred over LNF transfer when residual lymphatic channels to bypass can be identified preoperatively, because when properly planned and performed, LVA is a less invasive surgery than LNF with higher success rate if good channel can be found. LNF should be reserved to patients with lymphedema refractory to LVA or as first choice when no suitable residual lymphatic channels can be identified preoperatively.
Perioperative physical therapy is integral part of the treatment in order to boost the effect of microsurgical treatment.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Gianluca Franceschini, Alejandro Martín Sánchez, Riccardo Masetti) for the series “Update of Current Evidences in Breast Cancer Multidisciplinary Management” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.03.22). The series “Update of Current Evidences in Breast Cancer Multidisciplinary Management” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology. Lymphology 2013;46:1-11. [PubMed]
- Moffatt CJ, Franks PJ, Doherty DC, et al. Lymphoedema: an underestimated health problem. QJM 2003;96:731-8. [Crossref] [PubMed]
- Brayton KM, Hirsch AT, O, Brien PJ, et al. Lymphedema prevalence and treatment benefits in cancer: impact of a therapeutic intervention on health outcomes and costs. PLoS One 2014;9:e114597 [Crossref] [PubMed]
- Shih YC, Xu Y, Cormier JN, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol 2009;27:2007-14. [Crossref] [PubMed]
- Stout NL, Pfalzer LA, Springer B, et al. Breast cancer-related lymphedema: comparing direct costs of a prospective surveillance model and a traditional model of care. Phys Ther 2012;92:152-63. [Crossref] [PubMed]
- Kung TA, Champaneria MC, Maki JH, et al. Current Concepts in the Surgical Management of Lymphedema. Plast Reconstr Surg 2017;139:1003e-13e. [Crossref] [PubMed]
- Campisi C, Boccardo F. Frontiers in lymphatic microsurgery. Microsurgery 1998;18:462-71. [Crossref] [PubMed]
- Weiss M, Baumeister RG, Tatsch K, et al. Lymphoscintigraphy for noninvasive long term follow-up of functional outcome in patients with autologous lymph vessel transplantation. Nuklearmedizin 1996;35:236-42. [PubMed]
- Tartaglione G, Rubello D. The evolving methodology to perform limb lymphoscintigraphy: from rest to exercise acquisition protocol. Microvasc Res 2010;80:540-4. [Crossref] [PubMed]
- Tartaglione G, Pagan M, Morese R, et al. Intradermal lymphoscintigraphy at rest and after exercise: a new technique for the functional assessment of the lymphatic system in patients with lymphoedema. Nucl Med Commun 2010;31:547-51. [PubMed]
- Ogata F, Azuma R, Kikuchi M, et al. Novel lymphography using indocyanine green dye for near-infrared fluorescence labeling. Ann Plast Surg 2007;58:652-5. [Crossref] [PubMed]
- Narushima M, Yamamoto T, Ogata F, et al. Indocyanine green lymphography findings in limb lymphedema. J Reconstr Microsurg 2016;32:72-9. [PubMed]
- Yamamoto T, Narushima M, Doi K, et al. Characteristic indocyanine green lymphography findings in lower extremity lymphedema: the generation of a novel lymphedema severity staging system using dermal backflow patterns. Plast Reconstr Surg 2011;127:1979-86. [Crossref] [PubMed]
- Yamamoto T, Matsuda N, Doi K, et al. The earliest finding of indocyanine green (ICG) lymphography in asymptomatic limbs of lower extremity lymphedema patients secondary to cancer treatment: the modified dermal backflow (DB) stage and concept of subclinical lymphedema. Plast Reconstr Surg 2011;128:314e-21e. [Crossref] [PubMed]
- Yamamoto T, Yamamoto N, Doi K, et al. Indocyanine green (ICG)-enhanced lymphography for upper extremity lymphedema: a novel severity staging system using dermal backflow (DB) patterns. Plast Reconstr Surg 2011;128:941-7. [Crossref] [PubMed]
- Nawaz K, Hamad M, Sadek S, et al. Lymphscintigraphy in peripheral lymphedema using technetium-labelled human serum albumin: normal and abnormal patterns. Lymphology 1985;18:181-6. [PubMed]
- Suehiro K, Morikage N, Murakami M, et al. Re-evaluation of qualitative lymphangioscintigraphic findings in secondary lower extremity lymphedema. Surg Today 2014;44:1048-55. [Crossref] [PubMed]
- Szuba A, Shin WS, Strauss HW, et al. The third circulation: radionuclide lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med 2003;44:43-57. [PubMed]
- Maegawa J, Mikami T, Yamamoto Y, et al. Types of lymphoscintigraphy and indications for lymphaticovenous anastomosis. Microsurgery 2010;30:437-42. [Crossref] [PubMed]
- Blum KS, Radtke C, Knapp WH, et al. SPECT-CT: a valuable method to document the regeneration of lymphatics and autotransplanted lymph node fragments. Eur J Nucl Med Mol Imaging 2007;34:1861-7. [Crossref] [PubMed]
- Pecking AP, Wartski M, Cluzan RV, et al. SPECT-CT fusion imaging radionuclide lymphoscintigraphy: potential for limb lymphedema assessment and sentinel node detection in breast cancer. Cancer Treat Res 2007;135:79-84. [Crossref] [PubMed]
- Liu NF, Lu Q, Jiang ZH, et al. Anatomic and functional evaluation of the lymphatics and lymph nodes in diagnosis of lymphatic circulation disorders with contrast magnetic resonance lymphangiography. J Vasc Surg 2009;49:980-7. [Crossref] [PubMed]
- Liu NF, Yan ZX, Wu XF. Classification of lymphatic-system malformations in primary lymphoedema based on MR lymphangiography. Eur J Vasc Endovasc Surg 2012;44:345-9. [Crossref] [PubMed]
- Mitsumori LM, McDonald ES, Wilson GJ, et al. MR lymphangiography: How i do it. J Magn Reson Imaging 2015;42:1465-77. [Crossref] [PubMed]
- Adriaenssens N, Belsack D, Buyl R, et al. Ultrasound elastography as an objective diagnostic measurement tool for lymphedema of the treated breast in breast cancer patients following breast conserving surgery and radiotherapy. Radiol Oncol 2012;46:284-95. [Crossref] [PubMed]
- Hayashi N, Yamamoto T, Hayashi A, et al. Correlation between indocyanine green (ICG) patterns and real-time elastography images in lower extremity lymphedema patients. J Plast Reconstr Aesthet Surg 2015;68:1592-9. [Crossref] [PubMed]
- Hayashi A, Yamamoto T, Yoshimatsu H, et al. Ultrasound visualization of the lymphatic vessels in the lower leg. Microsurgery 2015;36:397-401. [Crossref] [PubMed]
- Visconti G, Yamamoto T, Hayashi N, et al. Ultrasound-Assisted Lymphaticovenular Anastomosis for the Treatment of Peripheral Lymphedema. Plast Reconstr Surg 2017;139:1380e-1e. [Crossref] [PubMed]
- Chang DW, Suami H, Skoracki R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast Reconstr Surg 2013;132:1305-14. [Crossref] [PubMed]
- Basta MN, Gao LL, Wu LC. Operative treatment of peripheral lymphedema: a systematic meta-analysis of the efficacy and safety of lymphovenous microsurgery and tissue transplantation. Plast Reconstr Surg 2014;133:905-13. [Crossref] [PubMed]
- Campisi CC, Ryan M, Boccardo F, et al. A Single-Site Technique of Multiple Lymphatic-Venous Anastomoses for the Treatment of Peripheral Lymphedema: Long-Term Clinical Outcome. J Reconstr Microsurg 2016;32:42-9. [PubMed]
- Koshima I, Inagawa K, Urushibara K, et al. Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J Reconstr Microsurg 2000;16:437-42. [Crossref] [PubMed]
- Ishiura R, Yamamoto T, Saito T, et al. Comparison of Lymphovenous Shunt Methods in a Rat Model: Supermicrosurgical Lymphaticovenular Anastomosis versus Microsurgical Lymphaticovenous Implantation. Plast Reconstr Surg 2017;139:1407-13. [Crossref] [PubMed]
- De Brucker B, Zeltzer A, Seidenstuecker K, et al. Breast Cancer-Related Lymphedema: Quality of Life after Lymph Node Transfer. Plast Reconstr Surg 2016;137:1673-80. [Crossref] [PubMed]
- Winters H, Tielemans HJ, Hameeteman M, et al. The efficacy of lymphaticovenular anastomosis in breast cancer-related lymphedema. Breast Cancer Res Treat. 2017;165:321-7. [Crossref] [PubMed]
- Patel NG, Ramakrishnan V. Microsurgical Tissue Transfer in Breast Reconstruction. Clin Plast Surg 2017;44:345-59. [Crossref] [PubMed]
- Chetta MD, Aliu O, Zhong L, et al. Reconstruction of the Irradiated Breast: A National Claims-Based Assessment of Postoperative Morbidity. Plast Reconstr Surg 2017;139:783-92. [Crossref] [PubMed]
- Cheng MH, Lin CY, Patel K. The Groin vs. Submental Vascularized Lymph Node Flaps: A Head-to-Head Comparison of Surgical Outcomes Following Treatment for Upper Limb Lymphedema. Plast Reconstr Surg 2015;136:135. [Crossref]
- Koshima I, Narushima M, Mihara M, et al. Lymphadiposal Flaps and Lymphaticovenular Anastomoses for Severe Leg Edema: Functional Reconstruction for Lymph Drainage System. J Reconstr Microsurg 2016;32:50-5. [PubMed]
- Mardonado AA, Chen R, Chang DW. The use of supraclavicular free flap with vascularized lymph node transfer for treatment of lymphedema: A prospective study of 100 consecutive cases. J Surg Oncol 2017;115:68-71. [Crossref] [PubMed]
- Saaristo AM, Niemi TS, Viitanen TP, et al. Microvascular breast reconstruction and lymph node transfer for postmastectomy lymphedema patients. Ann Surg 2012;255:468-73. [Crossref] [PubMed]
- Dayan JH, Dayan E, Smith ML. Reverse lymphatic mapping: a new technique for maximizing safety in vascularized lymph node transfer. Plast Reconstr Surg 2015;135:277-85. [Crossref] [PubMed]
- Koshima I, Yamamoto T, Narushima M, et al. Perforator flaps and supermicrosurgery. Clin Plast Surg 2010;37:683-9. [Crossref] [PubMed]
- Yamamoto T, Yoshimatsu H, Yamamoto N. Complete lymph flow reconstruction: A free vascularized lymph node true perforator flap transfer with efferent lymphaticolymphatic anastomosis. J Plast Reconstr Aesthet Surg 2016;69:1227-33. [Crossref] [PubMed]