
Current evidences on immediate breast reconstruction after mastectomy
Since the introduction of post mastectomy breast reconstruction in the 1980s, and the proposal of skin-sparing mastectomy, multiple refinements in reconstruction options, such as nipple-sparing mastectomy, have been introduced with enhanced results (1-9).
Patients with breast cancer need information about different breast reconstructive options available: a recent publication reported that about 50% of patients aim for breast reconstruction following mastectomy (10). Post-mastectomy breast reconstruction is related to important psychological benefit for patients. Information about timing of reconstruction is equally important: immediate reconstruction, at time of mastectomy, involves a much lower psychological effect than delayed reconstruction does. Several studies showed that in immediate breast reconstruction there is no delay to following adjuvant therapy; instead in patients with delayed reconstruction surgery is performed at least six months following the last adjuvant therapy.
Actually immediate breast reconstruction is the standard of cure; some discussions still exists as to whether immediate reconstruction should be offered to patients expected for postoperative radiotherapy.
Regardless of the indications, technique, or timing of reconstructive surgery, achieving a total breast reconstruction involves more procedures in order to obtain a desirable balance between the oncologic effectiveness and the maximization of the satisfaction with breast. The final goal of reconstructive breast surgery is to achieve a good shape similar to the original one. Following the works of Blondeel et al., the breast identity can be defined by the followings four elements (11):
- Breast footprint: the foundation of the overlying breast, including the inframammary fold (IMF);
- Breast conus: shape including ptosis, volume and projection. These mean the “contents” of the breast;
- Breast skin envelope: the “container”, what hold the breast “contents” on the footprint;
- Nipple-areola complex: usually placed on the point of maximal antero-posterior projection.
The breast conus is removed in every kind of mastectomy, being the parenchymal tissue its main component. The radical modified mastectomy removes also all the other three elements; skin-sparing mastectomy take off the conus and nipple-areola complex while saving the skin envelope and the IMF. The nipple-sparing mastectomy, as it is the more conservative one, preserves everything apart from the conus.
More elements are removed, more has to be replaced, therefore more demanding will be the reconstruction and the achievement of a pleasant breast.
The breast reconstructive surgery implies two different strategies to replace the breast conus: using prosthesis or using autologous tissue. The achievement of a suitable pocket is the key of success in breast reconstruction: if possible, the IMF, the pectoralis major muscle, and the overlying skin should be preserved. If any of these anatomical structures are removed, it should be reconstructed before introducing autologous tissue or an implant. The IMF should be repositioned and sutured in its original position. Shortage of mastectomy skin flaps has to be replaced either by autologous tissue or by preoperative expansion, in case of prosthetic reconstruction.
The reconstructive options after skin sparing mastectomy (SSM) and nipple sparing mastectomy (NSM) are one-stage or two-stage alloplastic reconstruction, and autologous tissues.
Prosthetic reconstruction
Prosthetic reconstruction still remains the most popular reconstructive procedure for its accessibility and quick execution. It gives excellent results especially in thin patients with small/medium breast and minimal ptosis, especially those patients desiring a breast augmentation; nevertheless, patients with medium/big size breast could also achieve good cosmetic results. Bilateral cases are encouraged because of symmetry, especially in the long term; in case of unilateral mastectomy, contralateral surgery is usually performed.
In patients with previous radiotherapy prosthetic reconstruction is discouraged.
Historically, reconstruction with implant implies the need of a two-stage procedure: an expander is positioned at time of mastectomy, which has to be replaced with a definitive prosthesis at least 6 months later. Even if nowadays the one-stage reconstruction is widespread, the use of skin expander is still suggested in some cases.
Since NSM has gained more popularity, one-stage prosthetic reconstruction has found largely space becoming a standard procedure in breast oncological surgery. One-stage reconstruction achieves direct to implant reconstruction at time of mastectomy and simultaneous contralateral symmetrization procedures, when needed, with a great patient’s psychological satisfaction.
In our series, results were found good to excellent results in 83% of cases of NSM and in 90% of cases of SSMs and SRM; therefore, one-stage immediate implant reconstruction is suggested. Prosthetic reconstruction, compared to autologous reconstruction is a simpler procedure with less morbidity and no donor site. Therefore, in our practice, we reserve autologous reconstruction after implant reconstruction failure and in patients previously irradiated or planned for radiation therapy.
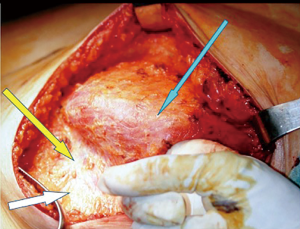
Our choice technique in immediate prosthetic breast reconstruction is positioning the prosthesis in a submuscular-subfascial pocket (12) (Figure 1).
The submuscular-subfascial pocket technique allows a good expansion of lower-pole tissue coverage, that, associated to redundancy of mastectomy flaps, consequent to conservative mastectomy, leads us to consider tissue expansion as really necessary just in few selected cases (12,13). Moreover, the undesirable skin retraction of the mastectomy flaps due to the placement of an expander would promote, in SSM and SRM, the nipple-areola complex lateralization (13).
On the other hand some authors are concerns in direct to implant reconstruction, because the skin stretching due to the definitive prosthesis could increase the risk of nipple-areola complex ischemia and skin flap sufferance.
Recent studies aimed to identify the risk of skin flap sufferance in breast reconstruction, have recognize the flap thickness as the crucial element in determining skin flap necrosis (14). The technique of flap dissection is critical to the viability of the mastectomy skin flaps: anatomically there is frequently a distinct layer of fatty tissue interposed between the lower dermal layer and parenchyma. The thickness of subcutaneous layer between dermis and parenchyma does not correlate with the body mass index of the patients or the breast sample weight. To maximize the viability and the cosmetic results of the breast reconstruction, the flap thickness should be as greater and the more uniform as possible. A recent study demonstrated that in 95% of women there is a subcutaneous layer of tissue between the caudal dermal layer and parenchyma that is about 1 cm thick (14).
An intraoperative angiography by indocyanine green (ICG) intravenous injection and infrared camera flap perfusion examination performed at the end of mastectomy could be used in controversial cases to verify the skin flap and nipple-areola complex viability therefore the reconstructive technique.
The submuscular-subfascial pocket is performed raising the pectoralis major muscle in continuity with adipofascial tissue which permits to obtain great expansion of the lower pole of the pocket immediately (12) (Figure 2).
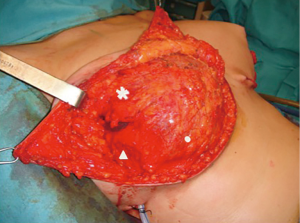
The subpectoral plane is reached by blunt incision of the lateral pectoralis fascia (LPF) in its superior part; the pectoralis muscle fibers aimed to the chest wall are cutted approaching the inferior part of the muscle. Then, the dissection is continued downward beneath the superficial pectoralis fascia (SPF) and the overlying subcutis, up to the intramammary fold. The lateral pectoral fascia (defined by the fusion of superficial pectoral fascia and deep pectoralis fascia lateral to pectoralis major) is elevated in continuity with the muscle to define the lateral part of the pocket. When this fascial layer is thin or slightly damaged, the serratus anterior muscle and its fascia is elevated.
The submuscular-subfascial pocket is mainly constituted by superficial structures of the thorax muscles, as follows (13):
- Superiorly by the released pectoralis major muscle (PM) with its fascias—deep pectoralis major fascia (DPF) and SPF;
- Inferiorly by the SPF;
- Laterally by the LPF and axillary fascia (AF) with or without the serratus anterior muscle (SA).
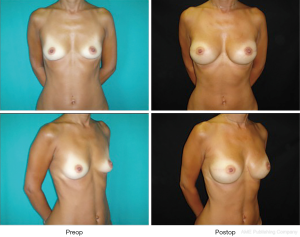
The musculofascial pocket has a great elasticity compared to a submuscular pocket allowing to host even a large prosthesis (up to 550 cc) while keeping an autologous cover of the implant (Figures 3,4). This peculiarity gains great importance in large breast where positioning a large volume implant could be challenging. Moreover, whenever a skin reducing mastectomy is required, complete implant coverage with viable tissue is recommended in areas at risk of breakdown, such as the T junction (15). This can help to minimize implant extrusion with consequent breast reconstruction failure.
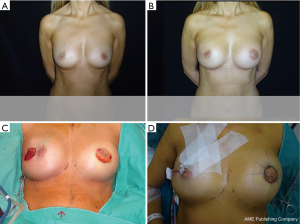
In cases when the SFS could not be preserved during mastectomy, or when the SPF is tougher than usual and in does not permit a good expansion of the lower pole, the one-stage breast reconstruction is performed by using synthetic and biological meshes such as the acellular dermal matrices (ADMs) (16-25).
The introduction of synthetic meshes and biological matrices has further changed the breast reconstruction.
A variety of ADMs are today available, derived from both allogenic and xenogenic donor sources. ADMs are more commonly used than synthetic meshes, and can derive from human, bovine and porcine sources. The technique for positioning the ADM/mesh is based on the complete elevation of pectoralis major muscle, the matrix is then placed in the lower pole of the breast sutured between the lower edge of the released muscle and the IMF, forming an internal bra to hold the definitive implant (Figure 5). With the advent of ADM, the submuscular placement of a definitive breast implant is performed in a single surgical procedure, achieving a more natural breast contouring (Figure 6).
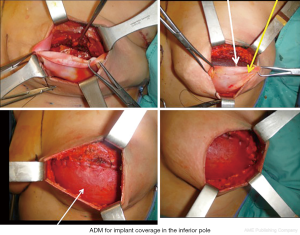
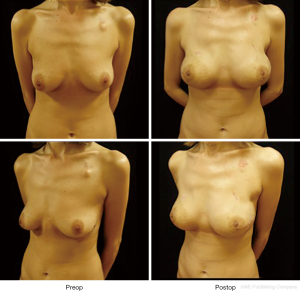
This technique has been widely popularized in the last years, as the conservative mastectomies became widespread, because of the advantages of a single stage reconstruction with improved lower pole projection that allows to achieve good cosmetic results even in difficult cases. Furthermore, Orenstein et al. demonstrated that in vitro ADM inhibits the production of interleukin (IL) and vascular endothelial growth factor thus reducing the incidence of capsular contracture in ADMs assisted breast reconstruction (26).
Despite the widespread use of ADMs, conflicting opinions concern the postoperative complications. Several papers associate the use of these ADMs with higher rate of postoperative seroma and infection, while other authors report no differences in complication rates if compared with the traditional expander-assisted breast reconstruction (27-29).
The limits of the sub-muscular techniques, either the submuscular-subfascial pocket or submuscular implant positioning together with the use of ADMs, seem to be consequent to the pectoralis major muscle release. Literature confirms that it weakens the muscle, thus altering the function of the shoulder joint and significantly impacting the daily activities. Physiotherapy rehabilitation after submuscular reconstruction could be demanding, and prolonged post-operative pain could be experienced (30,31).
In the attempt to optimize the breast reconstruction cosmetic outcomes with the use of ADMs, and to make the reconstructed even more breast natural, the prepectoral technique has been proposed (24,31) (Figure 7).
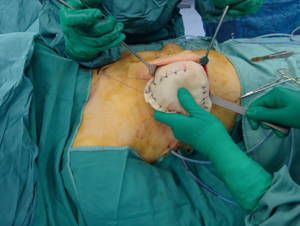
In fact, despite the physiological position of the mammary gland is over the pectoralis major muscle, the submuscular placement of the implant after mastectomy is commonly performed, which gives a static and artificial appearance to the reconstructed breast. Moreover, in case of unilateral mastectomy, an implant has to be positioned in the contralateral healthy breast to mimic the reconstructed breast appearance.
In selected cases, whenever the thickness of mastectomy flaps is kept to ensure a complete viability of the skin flap, the prosthesis is placed in the prepectoral plane, where the implant can be partially or totally covered by ADM. Prepectoral hosting of the implant is the physiological position. It avoids the postoperative complications related to the detachment of the pectoralis major muscle such as muscular pain and shoulder/arm weakness and faster recovery while improving the cosmetic outcomes.
The only pre-shaped ADM which completely wraps the implants is Braxon®. It is a porcine, 0.6 mm thick, non-cross-linked ADM with a patented shape, which fits implant ranging from 150 to about 500 cc (Figure 8). The implant is placed inside the matrix and completely wrapped around by suturing the matrix wings together with absorbable stitches to form a tight cover. The device is placed onto the pectoralis without detaching it and then anchored to the muscle with apical, medial and lateral absorbable stitches. ADM suturing is crucial to ensure primary stability and tight contact of the matrix with the vascularized tissue to allow complete integration. For this reason, quilting sutures between the ADM and the subcutaneous layer are recommended. Neovascularization and the inherent capacity of the biomaterial to be incorporated into the surrounding tissues provide a stable permanent cover.
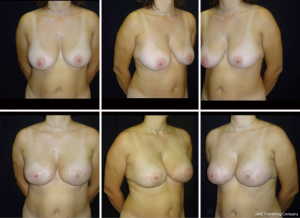
The prepectoral reconstructed breasts show natural movements, there is no evidence of breast animation deformities during pectoralis contraction. The shoulder movements are usually well-preserved and pain free, and the recovery after the surgery is easy, quick, and painless. Moreover, considering the latest findings about the use of ADM to reduce capsular fibrosis in human and primate models, total ADM coverage of the implant further reduces the incidence of capsular contracture as the ADM completely wraps the implant (32-34).
Autologous reconstruction
Autologous reconstruction techniques use tissues, skin and fat, and sometimes muscle, harvested from another place of the body to replace the breast conus and breast skin envelope, if needed. These tissues, called flaps, usually came from abdomen, buttocks, back, inner or external thigh to create the reconstructed breast. The tissue could be completely separated from its original source vessels, and they are called free as they are transferred and reconnected with microsurgery, or they can remain attached to its original source vessels, named pedicled.
It can be performed either after radical or conservative mastectomy. In case of unilateral mastectomy and autologous reconstruction, a good symmetry could be achieved with minimal or no surgery on the contralateral breast (Figure 9).
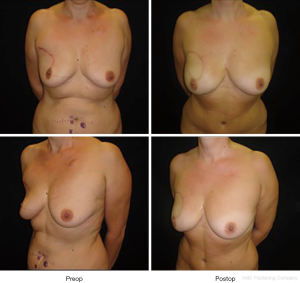
It gives excellent natural results especially in difficult cases which are difficulty amenable to implant reconstruction such as overweight patients with large breast and ptosis (35).
It’s a long lasting reconstruction, opposite to prosthetic reconstruction which experiences deterioration after same years. Moreover, the autologous breast reconstruction follows the body changes along the years also according to weight change.
Main indication to autologous reconstruction is previous radiotherapy.
In the last years, the microsurgical reconstructive options (free flaps recon) have gained a great diffusion worldwide not only in breast surgery, but also in other reconstructive fields, such as head and neck surgery and orthopedics; therefore, microsurgery is not confined any more just in few selected centers but has gained a lot of popularity even in small hospital.
The disadvantages of microsurgical flaps are the length of the procedure (usually longer than prosthetic reconstruction), and some complications that can drive to complete failure. Main complication is the thrombosis of the anastomosis: it can mainly occur in the first 72 hours, it is estimated around 3% of cases, and it requires a prompt return to the operating room to attempt to savage the flap. For this reason, strict monitoring of the flap in the first days is mandatory to quickly detect the changes in perfusion of the flap and promptly re-explore it. It results in a higher flap salvage rate as the severity of this phenomenon is correlated with the ischemia-time (36).
In planning microsurgical breast reconstruction preoperative screening is mandatory if the patient medical history suggests possible thrombophilia, as thrombophilia increase the risk of thrombotic complications in microvascular surgery (i.e., screening tests for antiphospholipid syndrome, hyperhomocysteinemia, factor V Leiden, protein S and C, activated protein C resistance, lupus anticoagulant, antithrombin, etc.). It is suggested in patients with history of deep venous thrombosis or with previous several miscarriages (37).
Flaps from abdominal area are the workhorse of autologous breast reconstruction because they can provide a large amount of well-vascularized tissue with minimal donor site morbidity. The abdominal flaps are adipocutaneous flaps and include deep inferior epigastric perforator (DIEP) flap and superficial inferior epigastric artery (SIEA) flap. The donor area is represented by the inferior part of the anterior abdominal area between the umbilicus and pubis, where western women usually have ample volume. Therefore, abdominal flaps can provide enough tissue to reconstruct large breast with a tension-free donor site closure. As the fat tissue from this area is soft and malleable, and its consistency resembles the natural consistency of breast tissue. it is suitable to mold the breast shape.
The vascular supply of the abdominal flaps can be provided by perforators of deep inferior epigastric pedicle (in DIEP flap) or by superficial inferior epigastric pedicle (in SIEA flap) (36).
Preoperative vascular studies performed by Doppler US, multidetector computer tomography, and magnetic resonance (38-42) have been proposed to study the dominant perforator/s to make this surgery more predictable. The dominant perforator, defined as the larger and centrally located perforator, is identified before surgery (39-42). The flap is elevated on the dominant perforator which is dissected in continuity with the inferior epigastric pedicle until its origins: the dissection spares the motor nerves, to keep the rectus abdominal muscle undamaged from anatomical and functional point of view to avoid bulges or hernias. The abdominal flap is tailored according with the volumetric needs of the breast recon. Peripheral areas of the flap, supposed to be not well vascularized, must be discharged according to the Holm classification (42). A clinical evaluation of the vascularization of peripheral area based on the bleeding color and intraoperative ICG angiography performed by infrared camera, permits to transfer the well vascularize tissue and to avoid complications such as liponecrosis and partial flap loss. Sometimes, when a very large flap is needed, additional venous anastomosis with superficial inferior vein. In these cases, distal stump of internal mammary vessels or thoracodorsal veins, cephalic vein or external jugular vein could be used as extra recipient vein (43). If a midline scar exists, or the patient needs a total abdominal flap, a bipedicle flap is required (44) (Figure 10).
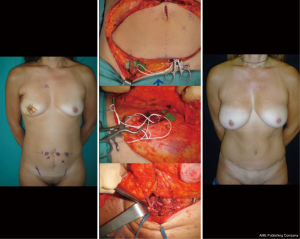
Alternately if, the preoperative vascular studies show a dominance of superficial inferior epigastric pedicles (less than 20% of patients), a SIEA flap can be raised. In this case, the dissection of the abdominal muscle and fascia is completely spared (abdominoplasty flap). Therefore, post-operative recovery is faster than in DIEP flap surgery and the risk of hernia is unreal (45).
For both flaps, the donor scar can be positioned adequately low so that it can be covered by normal underwear.
The flap, once raised, has to be anatomized to a recipient pedicle; for this reason, microsurgical approach is always performed by two teams: one prepares the donor site area including the dissection of recipient vessels; the second team performs the dissection the flap. The choice recipient vessels are represented by internal mammary vessels and thoracodorsal/circumflex scapula pedicles. The internal mammary vessels could be dissected by removing a small segment of second/third rib cartilage to provide a sufficient exposure or just in the second/third intercostal space in case of large one; it has the advantage to be centrally located in breast area with a comfortable exposure and it is usually not damaged by radiotherapy. It is described that internal mammary vein on the left side could be smaller than on the right side. At the level of second and third intercostal space, usually large perforators are located and could be used as recipient vessels for free flap in 10%/15% of cases.
Chest wall inflammation, as after infected prosthesis removal, or severe capsular fibrosis, or severe radiation therapy sequelae, can cause extreme perivascular scarring. Even if artery keeps almost always a good caliber, the size of the vein/veins is very variable.
Thoracodorsal pedicle is widely used as recipient vessels in breast reconstruction surgery especially in immediate setting, when axilla is dissected, because of the exposition of the vessels. In patient with previous radiotherapy and/or previous axillary dissection, the thoracodorsal vessels dissection could be tedious because of the perivascular scarring. In axilla region, the subscapular, the circumflex scapular and axillary vessels can also be used to accommodate a free flap; but in these cases, a longer flap’s pedicle is required to reach the recipient vessel and meanwhile to allow the flap’s shaping. Sometimes performing the anastomosis in the axilla could be troublesome.
There are also other microsurgical flaps available from other body’s areas for women who do not have enough tissue on their abdomen: Superior and inferior gluteal perforator flaps, thigh upper gracilis, profunda femoris flap, etc. They are considered as second choice options compared to abdominal flaps because of more visible scars and, in unilateral cases, resulting asymmetry due to flap raising. The pedicle of these flaps are shorter (on average 8 cm) then the DIEP flap pedicle, therefore anastomosis could be troublesome at times.
We consider latissimus dorsi (LD) breast reconstruction a suitable option in selected cases: mainly after microsurgical flap loss, or in patients discouraged to have microsurgical reconstruction. LD breast reconstruction uses a musculocutaneous flap from the back which is transferred to breast area, with or without the need of an implant. The raising of the LD flap leaves a great morbidity in the back region due to the muscle’ sacrifice consequent to flap mobilization. The advantage of LD breast reconstruction is no microanastomosis need because it is a pedicle flap; so it is an easier flap to be performed compared to the microsurgical flaps.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Gianluca Franceschini, Alejandro Martín Sánchez, Riccardo Masetti) for the series “Update of Current Evidences in Breast Cancer Multidisciplinary Management” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.03.40). The series “Update of Current Evidences in Breast Cancer Multidisciplinary Management” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vlajcic Z, Rado Z, Stanec S, et al. Nipple-areola complex preservation. Plast Reconstr Surg 2006;118:1493-5. [Crossref] [PubMed]
- Simmons RM, Hollenbeck ST, Latrenta GS. Areola-sparing mastectomy with immediate breast reconstruction. Ann Plast Surg 2003;51:547-51. [Crossref] [PubMed]
- Ma G, Richardson H, Pacella SJ, et al. Single-stage breast reconstruction following areola-sparing mastectomy. Plast Reconstr Surg 2009;123:1414-7. [Crossref] [PubMed]
- Margulies AG, Hochberg J, Kepple J, et al. Total skin-sparing mastectomy without preservation of the nipple-areola complex. Am J Surg 2005;190:907-12. [Crossref] [PubMed]
- Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy. Ann Surg 2009;249:26-32. [Crossref] [PubMed]
- Jensen JA. When can the nipple-areola complex safely be spared during mastectomy? Plast Reconstr Surg 2002;109:805-7. [Crossref] [PubMed]
- Gerber B, Krause A, Reimer T, et al. Skin-sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction is an oncologically safe procedure. Ann Surg 2003;238:120-7. [Crossref] [PubMed]
- Gerber B, Krause A, Dieterich M, et al. The oncological safety of skin-sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction: An extended follow-up study. Ann Surg 2009;249:461-8. [Crossref] [PubMed]
- Benediktsson KP, Perbeck L. Survival in breast cancer after nipple-sparing subcutaneous mastectomy and immediate re- construction with implants: A prospective trial with 13 years median follow-up in 216 patients. Eur J Surg Oncol 2008;34:143-8. [Crossref] [PubMed]
- Keith DJ, Walker MB, Walker LG, et al. Women who wish breast reconstruction. Charateristics, frear and hopes. Plast Reconstr Surg 2003;111:1051-6; discussion 1057-9. [Crossref] [PubMed]
- Blondeel PN, Hijjawi J, Depypere H, et al. Shaping the breast in aesthetic and reconstructive breast surgery: an easy three-step principle. Plast Reconstr Surg 2009;123:455-62. [Crossref] [PubMed]
- Salgarello M, Barone Adesi L, Terribile D, et al. Update on one-stage immediate reconstruction with definitive prosthesis after sparing mastectomies. Breast 2011;20:7-14. [Crossref] [PubMed]
- Salgarello M, Visconti G, Barone Adesi L. One-stage immediate reconstruction with implants in conservative mastectomies. Breast Reconstruction – Current Techniques. Published on: 2012-02-03.
- Robertson SA, Rusby JE, Cutress RI. Determinants of optimal mastectomy skin flap thickness. Br J Surg 2014;101:899-911. [Crossref] [PubMed]
- Salgarello M, Visconti G, Barone Adesi L, et al. Inverted-T skin-reducing mastectomy with immediate implant reconstruction using the submuscular- subfascial pocket. Plast Reconstr Surg 2012;130:31-41. [Crossref] [PubMed]
- Breuing KH. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg 2005;55:232Y239.
- Zienowicz RJ, Karacaoglu E. Implant-based breast reconstruction with allograft. Plast Reconstr Surg 2007;120:373-81. [Crossref] [PubMed]
- Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg 2006;57:1-5. [Crossref] [PubMed]
- Salzberg CA. Direct-to-implant breast reconstruction. Clin. Plast. Surg 2012;39:119-26. [Crossref] [PubMed]
- Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg 2011;127:514-24. [Crossref] [PubMed]
- Sbitany H, Sandeen SN, Amalfi AN, et al. Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Plast Reconstr Surg 2009;124:1735-40. [Crossref] [PubMed]
- Johnson RK, Wright CK, Gandhi A, et al. Cost minimisation analysis of using acellular dermal matrix (StratticeTM) for breast reconstruction compared with standard techniques. Eur J Surg Oncol 2013;39:242-7. [Crossref] [PubMed]
- Nahabedian MY. Acellular dermal matrices in primary breast recon- struction: principles, concepts, and indications. Plast Reconstr Surg 2012;130:44S-53S. [Crossref] [PubMed]
- Kiernan T, Martin L. Use of acellular dermal matrix is comparable to expander based breast reconstructions for post-operative physiotherapy requirements. Surg Curr Res 2013;3:136-7.
- McCarthy CM, Lee CN, Halvorson EG, et al. The use of acellular dermal matrices in two-stage expander/implant reconstruction: a multicenter, blinded, randomized controlled trial. Plast Reconstr Surg 2012;130:57S-66S. [Crossref] [PubMed]
- Orenstein SB, Qiao Y, Kaur M, et al. Human monocyte activation by biologic and biodegradable meshes in vitro. Surg Endosc 2010;24:805-11. [Crossref] [PubMed]
- Sbitany H, Serletti JM. Acellular dermis-assisted prosthetic breast reconstruction: a systematic and critical review of efficacy and associated morbidity. Plast Reconstr Surg 2011;128:1162-9. [Crossref] [PubMed]
- Hoppe IC, Yueh JH, Wei CH, et al. Complications Following Expander/Implant Breast Reconstruction Utilizing Acellular Dermal Matrix: A Systematic Review and Meta-Analysis. Eplasty 2011;11:e40 [PubMed]
- Davila AA, Seth AK, Wang E, et al. Human Acellular Dermis versus Submuscular Tissue Expander Breast Reconstruction: A Multivariate Analysis of Short-Term Complications. Arch Plast Surg 2013;40:19-27. [Crossref] [PubMed]
- Wallace MS, Wallace AM, et al. Pain after breast surgery: a survey of 282 women. Pain 1996;66:195-205. [Crossref] [PubMed]
- Berna G, Cawthorn SJ, Papaccio G, et al. Evaluation of a novel breast reconstructiontechnique using the Braxon® acellular dermal matrix: a new muscle-sparing breast reconstruction. ANZ J Surg 2017;87:493-8. [Crossref] [PubMed]
- Schmitz M, Bertram M, Kneser U, et al. Experimental total wrapping of breast implants with ADM: a preventive tool against capsular contracture in breast surgery? J Plast Reconstr Aesthet Surg 2013;66:1382-9. [Crossref] [PubMed]
- Cheng A, Lakhiani C, Saint-Cyr M, et al. Treatment of capsular contracture using complete implant coverage by ADM: a novel technique. Plast Reconstr Surg 2013;132:519-29. [Crossref] [PubMed]
- Vidya R, Masià J, Cawthorn S, et al. Evaluation of the effectiveness of the prepectoral breast recon with Braxon dermal matrix: 1 multicenter European report on 100 cases. Breast J 2017;23:670-6. [Crossref] [PubMed]
- Blondeel PN. One hundred free DIEP flap breast reconstructions: a personal experience. Br J Plast Surg 1999;52:104-11. [Crossref] [PubMed]
- Zoccali G, Molina A, Farhadi J. Is long term post-operative monitoring of microsurgial flaps still necessary? J Plast Reconstr Aesthet Surg 2017;70:996-1000. [Crossref] [PubMed]
- Pannucci CJ, Kovach SJ, Cuker A. Microsurgery and Hypercoagulable state. A hematologist’s perspective. Plast Reconstr Surg 2015;136:545e-52e. [Crossref] [PubMed]
- Blondeel PN, Beyens G, Verhaeghe R, et al. Doppler flowmetry in planning of perforator flaps. Br J plast Surg 1998;51:202-9. [Crossref] [PubMed]
- Masia J, Kosutic D, Clavero JA, et al. Preoperative computed tomographic angiogram for deep inferior epigastric artery perforator flap breast reconstruction. J Reconstr Microsurg 2010;26:21-8. [Crossref] [PubMed]
- Masia J, Navarro C, Clavero JA, et al. Noncontrast magnetic resonance imaging for preoperative perforator mapping. Clin Plast Surg 2011;38:253-61. [Crossref] [PubMed]
- Cina A, Barone-Adesi L, Rinaldi P, et al. Planning deep inferior epigastric perforator flaps for breast reconstruction: a comparison between multidetector computed tomography and magnetic resonance angiography. Eur Radiol 2013;23:2333-43. [Crossref] [PubMed]
- Holm C, Mayr M, Höfter E, et al. Perfusion zones of the DIEP flap revisited: a clinical study. Plast Reconstr Surg 2006;117:37-43. [Crossref] [PubMed]
- Salgarello M, Visconti G, Barone-Adesi L, et al. The retrograde limb of internal mammary vessels as reliable recipient vessels in DIEP flap breast reconstruction: a clinical and radiological study. Ann Plast Surg 2015;74:447-53. [Crossref] [PubMed]
- Hernandez Rosa J, Sherif RD, Torina PJ, et al. Use of both antegrade and retrograde internal mammary vessels in the bipedicled deep inferior epigastric perforator flap for unilateral breast reconstruction. J Plast Reconstr Aesthet Surg 2017;70:47-53. [Crossref] [PubMed]
- Knox AD, Ho AL, Leung L, et al. Comparison of Outcomes following Autologous Breast Reconstruction Using the DIEP and Pedicled TRAM Flaps: A 12-Year Clinical Retrospective Study and Literature Review. Plast Reconstr Surg 2016;138:16-28. [Crossref] [PubMed]


