
PD-1/PD-L1 pathway inhibition to restore effector functions in exhausted CD8+ T cells: chances, limitations and potential risks
Programmed cell death-1 (PD-1)/programmed death-ligand 1 (PD-L1) pathway
The PD-1 receptor is expressed at the surface of activated T cells. It has two known ligands, PD-L1 and PD-L2 (B7-DC). Its high affinity ligand PD-L1 is commonly expressed at the surface of hematopoietic cells such as dendritic cells (DCs) and macrophages as well as on non-hematopoietic cells such as epithelial and endothelial cells. In contrast, PD-L2 is mainly expressed on antigen presenting cells (APCs), DCs and macrophages (1,2). Both ligands are usually described as immune checkpoint proteins that act as co-inhibitory molecules and can terminate the effector phase of T cell-mediated immune responses (3). When PD-L1 binds to PD-1, an inhibitory signal is transmitted into the T cell that reduces cytokine production, suppresses T cell proliferation (1,4) and mainly incapacitates the cytotoxic and memory potential of CD8+ T cells (5-7). The homeostatic PD-1/PD-L1 interaction ensures immune activation for only an appropriate time in order to curtail the likelihood for the development of chronic inflammation. Accordingly, disruption of the PD-1 or PD-L1 gene aggravates T cell responses resulting in the generation of self-reactive T cells, development of lupus like disorder and dilated cardiomyopathy (8,9). However, PD-1/PD-L1 interaction represents a major drawback in certain other disease settings such as cancer or chronic viral infections, where PD-1 signalling in T cells impinges on anti-tumor or anti-viral immune responses. In particular, tumor cells or pathogens causing chronic infections exploit this immune checkpoint pathway of over-expressing PD-1 and PD-L1 as a mechanism to evade cytotoxic immune surveillance (10-12).
CD8+ T cell exhaustion and PD-1/PD-L1 pathway inhibition
CD8+ T cell exhaustion is characterized by the dysfunctionality of the cells to mount appropriate immune responses against tumor antigens or persisting pathogens. Generally, exhausted CD8+ T cells exhibit poor effector functions, which is associated with elevated expression of multiple sets of immune inhibitory receptors (13). The well-studied PD-1/PD-L1 receptor-ligand pair interaction inhibits the functional renewal of the CD8+ T cell repertoire in many human chronic diseases (14,15). The extracellular region of PD-1 consists of a single IgV-like domain and its cytoplasmic tail has two motifs, immunoreceptor tyrosine based inhibitory motif (ITIM) and immunoreceptor tyrosine based switch motif (ITSM) domain, respectively. ITIM and ITSM serve as docking sites for SH2 phosphatases that dephosphorylate and thereby inactivate ZAP-70, an integral component of T cell receptor–mediated signalling (16). This results in the inhibition of T cell proliferation and effector functions like cytotoxicity and IFN-γ secretion (17). Recently, it has been demonstrated that the PD-1-dependent Src Homology 2 (SH2) phosphatase preferentially dephosphorylates the cytoplasmic tail of the CD28 costimulatory receptor to potentiate T cell inhibition (18). Thus, blocking of the PD-1/PD-L1 pathway with antagonistic antibodies would substantially improve the functionality of exhausted cytotoxic CD8+ T cells. Such therapeutic intervention was successfully applied to boost CD8+ T cell reactivity to tumors and to invigorate the body’s own defensive mechanisms to remain effective against any malignancies. Food and Drug Administration (FDA) approved monoclonal antibody therapies targeting PD-1 (nivolumab and pembrolizumab) and PD-L1 (atezolizumab) revealed promising outcomes in clinical trials. Nivolumab is an anti-PD1 drug developed by Bristol-Myers Squibb, which is approved for metastatic melanoma and squamous non-small cell lung cancer (NSCLC) (19), while the PD-1 antagonist Pembrolizumab developed by Merck, is approved for metastatic melanoma (20). Atezolizumab is a new anti-PD-L1 drug developed by Roche and is approved for the treatment of patients with metastatic NSCLC (21) and metastatic urothelial carcinoma (22). The mechanisms of CD8+ T cell rejuvenation upon anti-PD-1 therapy is shown in Figure 1.
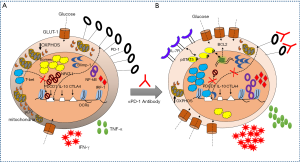
Hidden facts behind therapeutic PD-1/PD-L1 pathway inhibition
Despite tremendous collaborative efforts made by academic-industrial partners to develop monoclonal antibodies specifically inhibiting the PD-1/PD-L1 axis, a recent study has revealed some extraordinarily important facts that force us to re-consider the therapeutic concept underlying the targeting of the PD-1 signalling pathway during chronic diseases. Here, an in-depth comparison between αPD-L1 treated and untreated exhausted CD8+ T cells was done using unbiased transcriptional profiling and epigenomics. In addition, αPD-L1 treated and untreated exhausted CD8+ T cells were compared with functionally competent memory or effector CD8+ T cells, wherein αPD-L1 treated exhausted CD8+ T cells lost their recall ability, a hallmark feature of memory CD8+ T cells, and displayed an inflexible epigenome correlating with the repression of immune inhibitory genes like PDCD1, CTLA4 and IL10 (23).
Intriguingly, Pauken et al. has shown that αPD-L1 treatment induces the expression of interleukin-7 receptor (IL-7R) (CD127) on exhausted T cells during chronic lymphocytic choriomeningitis virus (LCMV) infection. PD-L1 induced IL-7R re-expression on exhausted CD8+ T cells may in turn render them responsive to the homeostatic cytokine IL-7 (23). Until now, the two best characterized features of IL-7 are that it acts as a pivotal factor for (I) the long-term survival of mature effector CD8+ T cells by inducing upregulation of the anti-apoptotic marker Bcl-2 (24) and (II) the generation of a memory T cell phenotype (25). Apart, IL-7 regulates multiple functions during chronic LCMV infection by (I) augmenting the co-production of effector cytokines such as IL-17, IL-6, IFN-γ; (II) inducing the secretion of the cytoprotective cytokine IL-22; (III) repressing the suppressor of cytokine signalling (SOCS3) and, importantly; (IV) down-regulating PD-1 expression to reinvigorate the effector functions of CD8+ T cells. According to data reported by Pauken et al. (23), the course of chronic LCMV infection following monotherapy with IL-7 alone was not positively affected, although the homeostatic proliferation of memory CD8+ T cells was indeed IL-7 dependent. In contrast, combined αPD-L1 and IL-7 therapy was shown to have additive effects resulting in the expansion of LCMV-specific CD8+ T cells co-producing IFN-γ and tumor necrosis factor-alpha (TNF-α). Apparently, IL-7 acts in concert with IL-15 to maintain Bcl-2 expression, which is involved in the phosphorylation of signal transducer and activator of transcription 5 (STAT-5) and thus supports the generation of long-lived effector CD8+ T cells (26). Although, an increased STAT-5 phosphorylation is observed in αPD-L1 treated exhausted CD8+ T cells upon additional IL-7 stimulation, Pauken et al. have noticed that the expression of the IL-15 receptor CD122 did not differ between αPD-L1 treated and untreated exhausted CD8+ T cells in the chronically LCMV infected host. This imbalance in the expression pattern of homeostatic cytokine receptors could possibly account for only a partially improved survival of αPD-L1 treated exhausted CD8+ T cells, even in an antigen-free environment (23). These data clearly pinpoint drastic changes that are occurring possibly at both transcriptional and epigenetic levels in exhausted CD8+ T cells and that are not sufficiently altered upon αPD-L1 treatment.
Transcription factors and PD-1 expression
Antigen-experienced cytotoxic CD8+ T cells are known to express the transcription factor eomesodermin (Eomes) (27) in addition to the T-box transcription factor (T-bet). Eomes+ CD8+ T cells exhibit characteristic features of memory cells (28), whereas T-bet+ CD8+ T cells are competent to execute effector functions (29). These transcription factors play reciprocal roles in fine-tuning the balance between terminally differentiated effector cells and self-renewing memory CD8+ T cells (30). During chronic viral infections, Eomeshi CD8+ T cells outnumber T-bethi CD8+ T cells in bone marrow, liver, gut, lung, skin, brain, blood, with varying proportions (31) and most importantly, the majority of exhausted CD8+ T cells is generally described to be T-betlow and Eomeshigh, despite some minor proportion of the exhausted CD8+ T cells exhibit a T-bethigh Eomeslow phenotype (32). Such Eomes-expressing CD8+ T cells display high expression of several inhibitory receptors, including PD-1, which is associated with a severe state of exhaustion (33,34). Furthermore, Eomeshigh virus-specific CD8+ T cells show reduced co-production of TNF-α and IFN-γ (32). Since T-bet expression was shown to directly repress the expression of PD-1, a substantial increase in Eomeshigh T-betlow antigen-specific CD8+ T cells loses its capability to suppress PD-1 expression of (35). In line with this, the transcription factor T-bet was shown to bind to the regulatory elements located upstream of the PDCD1 gene, which represents as well a binding site for the transcription factor nuclear factor of activated T cells (NFAT). NFAT generally enhances PDCD1 expression which explains the antagonism between T-bet and NFAT in terms of PD-1 expression (36). However, during the state of chronic infection direct T-bet-PDCD1 interaction could not be demonstrated, which may mainly be due to the overall low expression level of T-bet in exhausted CD8+ T cells. Reciprocal effects of Eomes and T-bet on PD-1 expression could, at least in part, also be explained by the localization of the transcription factors with high levels of Eomes in exhausted CD8+ T cells being associated with its nuclear localization and low levels of T-bet being associated with its cytoplasmic localization. In line with this, it has been reported that in effector CD8+ T cells 85% of T-bet is located in the nucleus (37), suggesting a role of T-bet in effector functions. This is further corroborated by reports stating that T-bethighEomeslowPD-1int exhausted CD8+ T cells are more susceptible to functional rescue by PD-1 blockade than T-betlowEomeshighPD-1highexhausted CD8+ T cells (23).
Despite having the potential for memory cell differentiation, these Eomeshigh exhausted CD8+ T cells fail to develop a recall response upon secondary antigenic challenge. This could be in a part explained by elevated expression of PD-1. On the other hand, it might be possible that Eomeshigh CD8+ T cells are paralyzed since they lack help from CD4+ T cells, which also express high levels of PD-1 during chronic infection (38,39).
Epigenetic changes in exhausted T cells
Chronic antigenic stimulation is associated with unique changes in the chromatin accessibility in exhausted CD8+ T cells. During chronic LCMV infection, an enrichment of open chromatin regions (OCRs), which is generally associated with transcriptional activation, was observed in the vicinity of immune-inhibitory genes such as PDCD1, IL10, and CTLA4 in exhausted T cells. αPD-L1 treatment did not affect these modifications, indicating a relatively stable epigenetic imprinting in exhausted CD8+ T cells. Moreover, almost 6,000 OCRs modifications were uncovered to be unique for the exhausted T cell phenotype when compared to effector or memory T cells implying that exhausted T cells may develop into a distinct T cell lineage due to their rigid epigenome. Upon PD-1 pathway blockade, almost 650 OCRs modification were induced. More careful exploration revealed that αPD-L1 treated exhausted T cells exhibit considerable changes in a set of genes located close to OCRs containing effector cell-related transcription factors-binding motifs, wherein the activity of NF-κB, IRF-1 and Blimp-1 was augmented. At the same time, a reduced expression of the genes located within OCRs encoding for T cell exhaustion-promoting transcription factors such as NFAT, NFAT-AP-1, IRF-4 and BATF was observed upon αPD-L1 treatment of exhausted CD8+ T cells. Thus, the notion behind the PD-L1 blockade implicates rewired transcriptional activity to re-establish the expression of effector genes within non-reprogrammable PD-1hi dysfunctional CD8+ T cells (23).
Blimp-1, one of the effector molecules that was induced in exhausted CD8+ T cells upon PD-L1 blockade, was reported to act directly on the PDCD1 gene locus by supporting the formation of a closed chromatin structure and thereby preventing direct binding of NFATc1 (40). Moreover, Blimp-1 supports the expansion of perforin+ granzyme+ cytolytic CD8+ effector T cells, whereas the formation of memory precursor cells is hindered. Here, Blimp-1 binds to the promoter region of Id3 to repress its expression in effector CD8+ T cells. The reduced expression of Id3 triggers CD8+ effector T cell death and thereby limits memory cell development (41). This could explain the mechanism underlying the defective recall response by memory T cells during re-infection upon αPD-L1 blockade. Likewise, NF-κB is considered to be a critical factor for the survival of proliferating CD8+ T cells (42). Furthermore, PD-L1 blockade augments the activity of IRF-1 in exhausted CD8+ T cells. Likewise, the therapy might also modulate PD-L1 reverse signalling activities inside APCs. It has been reported that the expression of IRF-1 as well as NF-κB in APCS are considered to be pivotal for the antigen processing machinery that is needed for the proper expression of MHC-I molecules on APCs, thereby rendering them susceptible to CD8+ T cell mediated cytotoxicity (43). In particular, IRF-1 deficient mice exhibit impaired expression of TAP and LMP-2 genes. Since these molecules are crucial for MHC-I-dependent antigen presentation, IRF-1 deficiency contributes to the reduction of CD8+ T cell numbers (44). Though IRF-1 is a regulator of type I interferon expression during viral infections, it is also a well-characterized factor known to suppress the tumor activity as evidenced by an early onset tumorigenesis, increased tumor burden and multiplicity, and striking changes in the tumor spectrum in mice lacking the IRF-1 gene (45). In addition, tumor-derived lysosomes containing IRF-1 also enhance the recruitment of CD8+ T cells to the tumor microenvironment and mediate antitumor immune responses (46). Thus, such shift in the potential effector-related transcriptional factor motif enrichments in the chromatin could act as a predictive marker for the ability of infection or cancer-associated diseases to respond to the PD-L1 blockade therapy.
Metabolic reprogramming and PD-1/PD-L1 pathway inhibition
During chronic disease, the functional exhaustion of antigen-specific CD8+ T cells is reported to be directly linked to metabolic dysfunction. In fact, the early developmental stage of exhausted CD8+ T cells is mainly due to metabolic alterations. The transition of activated to exhausted CD8+ T cells is linked to reduced expression of glucose transporter-1 (GLUT-1) that is needed for glucose uptake into the cells and therefore serves as a limiting factor for glucose utilization (47,48). Moreover, PD-1 expressing CD8+ exhausted T cells also display dysregulated mitochondrial metabolism, which includes increased mitochondrial mass with depolarized mitochondria and increased production of ROS. It is well-known that a proper mitochondrial membrane potential is needed for the production of ATP during the process of oxidative phosphorylation (OXPHOS). During the early state of T cell exhaustion, mitochondrial dysfunction results in reduced OXPHOS and eventually reduced energy production. Mitochondria depolarization and dysfunction is mainly driven by the mammalian target of rapamycin (mTOR) signalling pathway that induces anabolic mitochondrial programming under glucose deprived conditions (48).
Intriguingly, it has been reported that the metabolic activity differs between PD-1hi and PD-1Int exhausted CD8+ T cells. Of note, a PD-1hi subset of exhausted T cells exhibits decreased glucose uptake, a reduced glycolytic and OXPHOS metabolism and higher mitochondrial mass with increased mTOR activity. This clearly implies a distinct role of PD-1 in the metabolic programing of exhausted T cells. The mechanistic link between PD-1 and metabolic modulation in CD8+ exhausted T cells is mainly due to the repression of the co-activator of peroxisomes proliferator- activator receptor gamma (PGC-1a), a key transcriptional regulator of genes controlling mitochondrial biogenesis and energy metabolism. Thus, metabolic reprogramming is equally important for the effector function of exhausted T cells (48).
PD-1/PD-L1 pathway inhibition: perspectives
Targeting PD-1/PD-L1 pathway represents an ideal tool to reactivate exhausted CD8+ T cells. PD-L1 does not only represent a ligand for PD-1 but also binds to CD80 on T cells, thereby inducing the transmission of negative signals to the T cells, resulting in reduced T cell proliferation and impaired IL-2 and IFN-γ production. Moreover, co-targeting of other inhibitory elements such as CTLA-4 or IL-10 cytokine, that are unaffected during the PD-1/PD-L1 pathway inhibition should be considered for effective reactivation of exhausted CD8+ T cells. In addition, treatment with an agonistic αCD28 antibody together with PD-L1 blockade might represent an attractive therapeutic approach for effective rejuvenation of exhausted T cells (49).
Reactivation of the expression of the transcription factors IRF-1 and NF-κB that was observed in exhausted CD8+ T cells upon PD-L1 blockade might possibly result in paradoxical outcomes. Despite IRF-1 is generally considered to be essential for antiviral defence inhibition of IRF-1 expression was shown to result in massive expansion of IFN-γ+ granzyme B+ antigen-specific effector CD8+ T cells. Cell intrinsic as well as cell extrinsic properties of IRF-1 were identified to contribute to shaping CD8+ T cell responses, including the IRF-1-dependent inhibiting of CD8+ T cell proliferation I addition to reducing the frequencies of regulatory T cells that under normal conditions restrain CD8+ T cell responses (50). Furthermore, IRF-1 and NF-κB retain the capability to induce PD-L1 expression on tumor cells (51,52). Apart, transcription factors such as IRF-4 and BATF, which were not re-expressed in exhausted CD8+ T cells following PD-L1 blockade, could also contribute to the overall maintainence of the effector function in CD8+ T cells, by increasing the IFN-γ production, the frequencies of antigen-specific CD8+ T cells and reducing the rate of apoptosis of CD8+ T cells during LCMV infection (53). PD-L1 blockade restores IRF-2 transcription factor expression that is best known for its ability to promote pro-oncogenic properties. Intriguingly, IRF-2 has been recently described to also possess a tumor-suppressive role, since silencing of the IRF-2 gene directly impairs the function of p53 (54). Hence, a careful consideration of the so far known consequences of αPD-L1 blockade with respect to the activation and repression of transcription factors, epigenetic modifications and metabolic reprogramming of exhausted CD8+ T cells is required to achieve a safe and beneficial reactivation of exhausted CD8+ T cells capable of conferring efficient effector function against persisting pathogens and tumors. In this context, co-targeting of the PD-1/PD-L1 pathway together with molecules of the IRF gene family or molecules related to metabolic pathways such as PGC-1a may have implications for the design of future therapeutics.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Xia Fang (Department of hematology, Shanghai Tongji Hospital, Tongji University School of Medicine, Shanghai, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.04.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027-34. [Crossref] [PubMed]
- Ishida M, Iwai Y, Tanaka Y, et al. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol Lett 2002;84:57-62. [Crossref] [PubMed]
- Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001;2:261-8. [Crossref] [PubMed]
- Brown JA, Dorfman DM, Ma FR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol 2003;170:1257-66. [Crossref] [PubMed]
- Blank C, Brown I, Peterson AC, et al. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res 2004;64:1140-5. [Crossref] [PubMed]
- Petrovas C, Casazza JP, Brenchley JM, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med 2006;203:2281-92. [Crossref] [PubMed]
- Zhang JY, Zhang Z, Wang X, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood 2007;109:4671-8. [Crossref] [PubMed]
- Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999;11:141-51. [Crossref] [PubMed]
- Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001;291:319-22. [Crossref] [PubMed]
- Woo SR, Turnis ME, Goldberg MV, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 2012;72:917-27. [Crossref] [PubMed]
- Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002;99:12293-7. [Crossref] [PubMed]
- Akhmetzyanova I, Drabczyk M, Neff CP, et al. PD-L1 Expression on Retrovirus-Infected Cells Mediates Immune Escape from CD8+ T Cell Killing. PLoS Pathog 2015;11:e1005224 [Crossref] [PubMed]
- Takamura S, Tsuji-Kawahara S, Yagita H, et al. Premature terminal exhaustion of Friend virus-specific effector CD8+ T cells by rapid induction of multiple inhibitory receptors. J Immunol 2010;184:4696-707. [Crossref] [PubMed]
- Boni C, Fisicaro P, Valdatta C, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol 2007;81:4215-25. [Crossref] [PubMed]
- Penna A, Pilli M, Zerbini A, et al. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology 2007;45:588-601. [Crossref] [PubMed]
- Chemnitz JM, Parry RV, Nichols KE, et al. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 2004;173:945-54. [Crossref] [PubMed]
- Jurado JO, Alvarez IB, Pasquinelli V, et al. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J Immunol 2008;181:116-25. [Crossref] [PubMed]
- Hui E, Cheung J, Zhu J, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 2017;355:1428-433. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014;384:1109-17. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp DOAK Study Group, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018;391:748-57. [Crossref] [PubMed]
- Pauken KE, Sammons MA. Odorizzi PMet al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 2016;354:1160-5. [Crossref] [PubMed]
- Kaech SM, Tan JT, Wherry EJ, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol 2003;4:1191-98. [Crossref] [PubMed]
- Schluns KS, Kieper WC, Jameson SC, et al. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol 2000;1:426-32. [Crossref] [PubMed]
- Tripathi P, Kurtulus S, Wojciechowski S, et al. STAT5 is critical to maintain effector CD8+ T cell responses. J Immunol 2010;185:2116-24. [Crossref] [PubMed]
- Pearce EL, Mullen AC, Martins GA, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 2003;302:1041-3. [Crossref] [PubMed]
- Banerjee A, Gordon SM, Intlekofer AM, et al. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J Immunol 2010;185:4988-92. [Crossref] [PubMed]
- Joshi NS, Cui W, Chandele A, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 2007;27:281-95. [Crossref] [PubMed]
- Intlekofer AM, Takemoto N, Kao C, et al. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med 2007;204:2015-21. [Crossref] [PubMed]
- Paley MA, Kroy DC, Odorizzi PM, et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 2012;338:1220-5. [Crossref] [PubMed]
- Buggert M, Tauriainen J, Yamamoto T, et al. T-bet and Eomes are differentially linked to the exhausted phenotype of CD8+ T cells in HIV infection. PLoS Pathog 2014;10:e1004251 [Crossref] [PubMed]
- Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2009;10:29-37. [Crossref] [PubMed]
- Fuertes Marraco SA, Neubert NJ, Verdeil G, et al. Inhibitory Receptors Beyond T Cell Exhaustion. Front Immunol 2015;6:310. [Crossref] [PubMed]
- Kao Charlly, Oestreich Kenneth J, et al. T-bet represses expression of PD-1 and sustains virus-specific CD8 T cell responses during chronic infection. Nat Immunol 2011;12:663-71. [Crossref] [PubMed]
- Oestreich KJ, Yoon H, Ahmed R, et al. NFATc1 regulates PD-1 expression upon T cell activation. J Immunol 2008;181:4832-9. [Crossref] [PubMed]
- McLane LM, Banerjee PP, Cosma GL, et al. Differential localization of T-bet and Eomes in CD8 T cell memory populations. J Immunol 2013;190:3207-15. [Crossref] [PubMed]
- Cockerham LR, Jain V, Sinclair E, et al. Programmed death-1 expression on CD4+ and CD8+ T cells in treated and untreated HIV disease. AIDS 2014;28:1749-58. [Crossref] [PubMed]
- Zhang S, Zhang H, Zhao J, et al. The role of CD4 T cell help for CD8 CTL activation. Biochem Biophys Res Commun 2009;384:405-8. [Crossref] [PubMed]
- Lu P, Youngblood BA, Austin JW, et al. Blimp-1 represses CD8 T cell expression of PD-1 using a feed-forward transcriptional circuit during acute viral infection. J Exp Med 2014;211:515-27. [Crossref] [PubMed]
- Ji Y, Pos Z, Rao M, et al. Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8+ T cells. Nat Immunol 2011;12:1230-37. [Crossref] [PubMed]
- Mondor I, Schmitt-Verhulst AM, Guerder S. RelA regulates the survival of activated effector CD8 T cells. Cell Death Differ 2005;12:1398-406. [Crossref] [PubMed]
- Lorenzi S, Forloni M, Cifaldi L, et al. IRF1 and NF-kB restore MHC class I-restricted tumor antigen processing and presentation to cytotoxic T cells in aggressive neuroblastoma. PLoS One 2012;7:e46928 [Crossref] [PubMed]
- White LC, Wright KL, Felix NJ, et al. Regulation of LMP2 and TAP1 genes by IRF-1 explains the paucity of CD8+ T cells in IRF-1-/- mice. Immunity 1996;5:365-76. [Crossref] [PubMed]
- Nozawa H, Oda E, Nakao K, et al. Loss of transcription factor IRF-1 affects tumor susceptibility in mice carrying the Ha-ras transgene or nullizygosity for p53. Genes Dev 1999;13:1240-5. [Crossref] [PubMed]
- Yang MQ, Du Q, Varley PR, et al. Interferon regulatory factor 1 priming of tumour-derived exosomes enhances the antitumour immune response. Br J Cancer 2018;118:62-71. [Crossref] [PubMed]
- Patsoukis N, Bardhan K, Chatterjee P, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun 2015;6:6692. [Crossref] [PubMed]
- Bengsch B, Johnson AL, Kurachi M, et al. Bioenergetic Insufficiencies Due to Metabolic Alterations Regulated by the Inhibitory Receptor PD-1 Are an Early Driver of CD8(+) T Cell Exhaustion. Immunity 2016;45:358-73. [Crossref] [PubMed]
- Kamphorst AO, Wieland A, Nasti T, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 2017;355:1423-7. [Crossref] [PubMed]
- Brien JD, Daffis S, Lazear HM, et al. Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8+ T Cell Immune Responses against West Nile Virus Infection. PLoS Pathog 2011;7:e1002230 [Crossref] [PubMed]
- Lee SJ, Jang BC, Lee SW, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274). FEBS Lett 2006;580:755-62. [Crossref] [PubMed]
- Garcia-Diaz A, Shin DS, Moreno BH, et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep 2017;19:1189-201. [Crossref] [PubMed]
- Grusdat M, McIlwain DR, Xu HC, et al. IRF4 and BATF are critical for CD8+ T-cell function following infection with LCMV. Cell Death Differ 2014;21:1050-60. [Crossref] [PubMed]
- Guichard C, Amaddeo G, Imbeaud S, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet 2012;44:694-98. [Crossref] [PubMed]


