Atezolizumab: state of art and future perspective in non-small cell lung cancer treatment
Lung cancer is the first cause of cancer deaths worldwide (1). Nowadays, treatment landscape of non-small cell lung cancer (NSCLC) is rapidly changing by the introduction of immune-checkpoint inhibitors (2,3). Programmed death-1 (PD-1), a key immune-checkpoint receptor expressed by activated T cells, down-regulates immune responses through binding to its ligand, programmed death ligand-1 (PD-L1). PD-L1 is expressed by tumor cells (TCs), tumor-infiltrating immune cells (ICs), or both (4). The US Food and Drug Administration (FDA) approved three immune-checkpoint inhibitors, the two anti-PD-1 nivolumab and pembrolizumab and the anti-PD-L1 atezolizumab, with substantial improvement in the management of NSCLC, both in first- (5) and second-line setting (2,3,6-8).
Pembrolizumab is the current front-line treatment for patients with PD-L1 strongly positive (>50%) advanced NSCLC based on the results of the phase III Keynote 024 trial (5). The study showed significant improvement in terms of overall response rate (ORR), progression free survival (PFS) and overall survival (OS) for patients receiving pembrolizumab compared to individuals treated with conventional platinum doublets. Interestingly, different results were observed in the Checkmate 026, a phase III study comparing conventional chemotherapy to nivolumab in NSCLC patients expressing PD-L1 ≥1%. The study failed to demonstrate any superiority of nivolumab over chemotherapy, highlighting the relevance of PD-L1 testing for selection of patients candidate to immunotherapy (9), at least in front line setting.
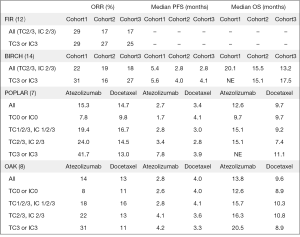
Atezolizumab, a humanized IgG1 monoclonal anti-PD-L1 antibody, binds PD-L1 and inhibits PD-L1-mediated signaling. The drug demonstrated efficacy in various solid tumors and it was first approved for the treatment of patients with locally advanced or metastatic urothelial carcinoma who progressed to front-line platinum-containing chemotherapy or with urothelial bladder cancer unsuitable for cisplatin (10,11). Moreover, this agent is the first anti-PD-L1 antibody demonstrating efficacy in pre-treated advanced NSCLC patients (7,8). Two international, randomized, open-label trials, the phase II POPLAR (7) and the phase III OAK study (8), clearly demonstrated the efficacy and safety of the drug when used in second line setting, leading to FDA and, more recently, European Medicines Agency (EMA) approval. In the POPLAR trial, comparing atezolizumab to docetaxel in NSCLC patients who failed platinum-doublet chemotherapy, atezolizumab prolonged median OS [12.6 vs. 9.7 months; hazard ratio (HR) =0.73; P=0.04] and median duration of response (DoR, 14.3 vs. 7.2 months), with no difference in terms of PFS (2.7 vs. 3.0 months) and ORR (15% in both arms) (7). In the trial, PD-L1 expression levels in both TCs and tumor-infiltrating ICs were evaluated by immunohistochemistry (IHC) using Ventana PD-L1 SP142 clone. Interestingly, the greatest benefit in terms of response and survival was observed in patients with high levels of PD-L1 expression receiving atezolizumab (ORR: 38% vs. 8%; OS: 15.5 vs. 11.1 months, P=0.068). These results were further replicated in the phase III OAK trial. In the study, patients treated with atezolizumab lived longer than those treated with docetaxel (13.8 vs. 9.6 months; HR =0.73; P=0.0003), even in absence of any improvements in terms of ORR or PFS. Patients enrolled were also stratified according to PD-L1 expression levels in TCs and ICs using the SP-142 IHC assay. Notably, survival was improved irrespective of PD-L1 expression, as demonstrated in PD-L1 low or undetectable population defined as TC0 and IC0 according to Ventana PD-L1 SP142 clone scoring system (OS was 12.6 vs. 8.9 months in atezolizumab and docetaxel arm respectively; HR =0.75, P=0.0215). However, patients categorized as TC3/IC3 (PD-L1 expression on TCs 50% or 10% on ICs) performed best (OS HR =0.41) (8). Another trial, the phase II FIR, explored the activity of atezolizumab as initial therapy in NSCLC with moderate or high PD-L1 expression (TC2/3 or IC 2/3) reporting an ORR of 29% and an OS was 9.5 months (12). More recently, Roche announced that phase III IMpower150 met its primary endpoint, demonstrating that addition of atezolizumab to front line carboplatin/paclitaxel/bevacizumab combination reduced the risk of progression in PD-L1 unselected NSCLC (13).
In a recent issue of The Journal of Clinical Oncology, Peters and colleagues reported results of the BIRCH trial, a phase II, single-arm, multicenter trial designed to assess the efficacy and safety of atezolizumab as first or subsequent line of therapy in patients with PD-L1 selected, advanced NSCLC (14). Notably, PD-L1 expression was evaluated and scored using the same system of the POPLAR and OAK trials. Overall, 667 patients were divided into three cohorts: cohort 1 chemotherapy-naïve patients (1L), cohort 2 one prior platinum-based regimen (2L), cohort 3 at least two prior chemotherapy regimens (3L). Patients allocated in cohort 1 received atezolizumab until disease progression or unacceptable toxicity, whereas in cohorts 2 or 3 patients were allowed to continue beyond progression at investigator’s discretion. The study met its primary end-point of significant ORR versus historical controls; independent review facility (IRF)-assessed ORR was 22% for cohort 1, 19% for cohort 2 and 18% for cohort 3. As expected, ORR was higher in the PD-L1 TC3 (50%) or IC3 (10%) subgroups (31%, 26%, 27%, respectively). Median DoR was 9.8 months, not estimable and 11.8 months and median PFS was 5.4, 2.8 and 2.8 months, for 1L, 2L and 3L, respectively. With the exception of 1L, in the other two cohorts PFS correlated with PD-L1 expression levels (2.6 months in overall population vs. 4.0 months in TC3/IC3 subgroup in cohort 2; 2.7 vs. 4.1 months, respectively, in cohort 3). Median OS was 23.5 months for chemotherapy-naïve patients, 15.5 months for patients treated in second-line and 13.2 months for patient treated in third line. The OS benefit was maintained in all cohorts and seemed independent of PD-L1 expression (26.9 in 1L, 16.6 in 2L and 17.5 months in 3L in TC3/IC3 subgroup). Interestingly, 8% of the entirely population harbored Epidermal growth factor receptor (EGFR) mutations and responses occurred both in wild type and EGFR mutated NSCLC across the three cohorts. Safety profile was consistent with previous studies and most common serious adverse events (AEs) were pneumonia (4%), dyspnea (3%), pneumonitis (2%), with 7% of patients discontinuing immunotherapy due to toxicity. These results confirmed that atezolizumab treatment derives in a clinical meaningful OS improvement in relation to chemotherapy historical controls. In addition, the BIRCH trial first showed the clinical benefit of this agent in first line PD-L1 selected advanced NSCLC, confirming preliminary data of the FIR trial (12), with an OS of approximately 2 years. Recently at World Conference on Lung Cancer 2017, Carcereny and colleagues presented updated efficacy results from the 138 patients in cohort 1 at a median follow-up of nearly 3 years. In all population ORR, PFS and OS were 26%, 7.6 and 24.0 months, respectively. Among the patients with TC3/IC3 PD-L1 expression (47% of all patients), ORR was 35%, PFS 7.3 months and OS was 26.9 months (15).
Take into account BIRCH findings reinforce two assumptions. First, immunotherapy is more effective when given early during the course of the disease. Hence, also in the Keynote 024 trial, PFS2 calculated from trial inclusion to failure of the second-line therapy significantly favored patients receiving immunotherapy first compared to the opposite sequence of chemo followed by immunotherapy (18.3 vs. 8.4 months; HR =0.54; P<0.001) (16). Second, immunotherapy is effective in pretreated NSCLC regardless PD-L1 expression, as demonstrated in previous trials (2,6,8). Data from the POPLAR, BIRCH and OAK clearly establish atezolizumab as a valid therapeutic option to offer in second or further line (7,8,14). Main results from atezolizumab studies are reported in Figure 1.
However, the efficacy of atezolizumab deserves confirmation in some special populations including patients with brain metastases (BMs) or with oncogene addicted NSCLC. As presence of BMs was a key exclusion criterion for BIRCH, the only available data come from the OAK trial in which patients with treated asymptomatic supratentorial BMs were eligible. Although in the subgroup of 85 patients with BM seemed to derive benefit from atezolizumab treatment (HR =0.54; 95% CI, 0.31–0.94, P=0.0279) (8), the optimal positioning of immunotherapy in the management of intracranial disease remains an open question. Similarly, there is no consensus on the role of immunotherapy in EGFR mutated NSCLC, as in general agreement oncogene addicted NSCLC are considered poor responders (2,3,8,17). Although data from BIRCH suggested a potential benefit even in presence of EGFR mutation, the small number precluded any conclusions.
In conclusion, data from all available trials justify the approval of atezolizumab in the second- or further-lines treatment regardless of PD-L1 expression. Others biomarkers should be further investigated to refine selection of patients candidate to second line immunotherapy. On the other hand, combination of atezolizumab and chemotherapy could become a new treatment option in first-line advanced NSCLC, given the definitive results from phase III IMpower150 trial.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research. The article did not undergo external peer review.
Conflicts of Interest: Dr. Cappuzzo declares consultancy role for Roche, Pfizer, Novartis and Takeda. Other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20. [Crossref] [PubMed]
- Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67-76. [Crossref] [PubMed]
- Spigel DR, Gettinger SN, Horn L, et al. Clinical activity and safety from a phase II study (FIR) of MPDL3280A (anti-PDL1) in PD-L1–selected patients with non-small cell lung cancer (NSCLC). J Clin Oncol 2015;33:8028.
- Phase III IMpower150 study showed Roche’s TECENTRIQ (atezolizumab) and Avastin (bevacizumab) plus carboplatin and paclitaxel helped people with advanced lung cancer live longer compared to Avastin plus carboplatin and paclitaxel. Available online: https://www.roche.com/media/store/releases/med-cor-2018-03-26.htm
- Peters S, Gettinger S, Johnson ML, et al. Phase II Trial of Atezolizumab As First-Line or Subsequent Therapy for Patients With Programmed Death-Ligand 1-Selected Advanced Non-Small-Cell Lung Cancer (BIRCH). J Clin Oncol 2017;35:2781-9. [Crossref] [PubMed]
- Carcereny E, Felip E, Reck M, et al. Updated efficacy results from the BIRCH study: first-line atezolizumab therapy in PD-L1-selected patients with advanced NSCLC. Presented at: International Association for the Study of Lung Cancer 18th World Conference on Lung Cancer; Yokohama, Japan: 2017. Abstract OA 17.02.
- Brahmer JR, Rodriguez-Abreu D, Robinson AG, et al. Progression after the next line of therapy (PFS2) and updated OS among patients (pts) with advanced NSCLC and PD-L1 tumor proportion score (TPS) ≥50% enrolled in KEYNOTE-024. J Clin Oncol 2017;35:9000.
- Lee CK, Man J, Lord S, et al. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol 2017;12:403-7. [Crossref] [PubMed]