
Sparing chemotherapy with dual HER2 blockade in combination with endocrine therapy for advanced HER2 positive breast cancer
The ALTERNATIVE trial has shown progression-free survival (PFS) benefit with the addition of lapatinib (LAP) to trastuzumab (TRAS) and an aromatase inhibitor (AI) among postmenopausal patients with hormone receptor (HR), human epidermal growth factor receptor 2 (HER2)-positive, metastatic breast cancer (MBC). These findings were reported by Johnston et al. in the Journal of Clinical Oncology (1).
HER-2 positive breast cancer represents approximately 20% of breast tumors, with substantial clinical and molecular heterogeneity (2,3). This heterogeneity has been suggested to underlie the variability of response and clinical benefit not only to anti-HER2 agents but also to endocrine therapies like tamoxifen and AIs.
Among HER-2 positive tumors, the level of ER expression apparently defines two distinct subtypes, with different biological behavior and different patterns of response and resistance to systemic anticancer therapies. The “triple positive” subtype, namely, estrogen receptor (ER)/progesterone receptor (PgR)/HER2 positive tumors that represent about 10% of all breast cancers, could be regarded as the subset which most closely resembles the luminal-like tumors. Nevertheless, it has been consistently reported that with HER2-positive/HR-positive tumors derive less benefit from endocrine therapy (ET) than patients with HER2-negative/HR-positive disease (4,5).
There is abundant preclinical evidence for a cross-talk between endocrine signaling and growth factor receptor (GFR) pathways, including HER family receptors (6,7). Retrospective studies showed that tumors of high HER2 expression levels are less responsive to treatment with tamoxifen possibly due to activation of downstream kinases, which turn increase the ER pathway transcriptional activity even in the presence of tamoxifen (8). Moreover, the nongenomic activation of the ER pathway can be activated by high levels of GFRs and their ligands (9). Extensive research has shown that enhanced expression of HER2 with increased activation of downstream signalization cascades by MAPK and PI3K/AKT/mTOR is associated with acquired resistance to ET (10).
This bidirectional crosstalk between ER and HER2 pathways possibly contributes not only to resistance to endocrine agents but also to anti-HER2 therapy (9). Preclinical studies hypothesized that some HER2-overexpressing tumors that are apparently ER-negative might actually revert to ER positivity after exposure to anti-HER2 agents (11). Therefore, simultaneous targeting of both HER2 and ER pathways is probably more effective than endocrine monotherapy. From this point of view, in addition to the interest in the PI3K/AKT/mTOR pathway, the inhibition of CDK4/6 represent a promising therapeutic strategy that will act downstream of the GFR pathways at the interface of proliferation signaling pathways and the cell cycle machinery (12).
The ALTERNATIVE trial is an open-label study in which eligible patients (from 122 sites in 29 countries) were randomly assigned (1:1:1) to treatment with LAP in combination with TRAS or TRAS or LAP. It is essential to emphasize that patients had received prior ET and previous neoadjuvant, adjuvant or first-line TRAS plus chemotherapy. LAP was given at 1,000 mg/day in the LAP-plus-TRAS group and at 1,500 mg/day in the LAP group. TRAS was given at the usual dose every 3 weeks. Investigator’s choice of AI included letrozole, anastrozole, or exemestane. The primary endpoint was investigator-assessed PFS for the combination versus the TRAS group in the intent-to-treat population.
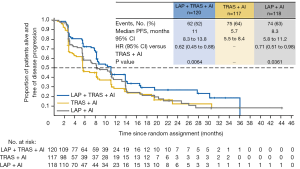
Median PFS was 11.0 months in the combination (LAP + TRAS + AI) group vs. 5.7 months in the TRAS (TRAS + AI) group (hazard ratio =0.62, P=0.0064) (see Figure 1). A consistent benefit of the combination treatment was observed across prespecified subgroups. Median PFS in the LAP group was 8.3 months (hazard ratio vs. TRAS group =0.71, P=0.0361). Overall response rates (ORR) in the combination, TRAS, and LAP groups were 31.7%, 13.7%, and 18.6%, with median durations of response of 13.9, 8.3, and 11.1 months, respectively. Overall survival (OS) data were immature at the time of analysis; median OS was 46.0, 40.0, and 45.1 months in the three groups.
The safety and toxicity profile of the three treatment arms was consistent with the known safety profile of TRAS and LAP. The frequency of adverse effects (AEs), mostly grade 1 or 2, was higher in the two LAP arms (92% each) than in the TRAS arm (74%). The most frequent AEs were diarrhea, rash, paronychia and nausea. Nonetheless, the incidence of grade 3 or 4 AEs and serious adverse events (SAEs) was similar in the three treatment groups, with the exception of grade 3 diarrhea, which was higher in the LAP + TRAS arm (13%).
Two previous trials have reported benefit of combining single HER2 blockade plus ET, without chemotherapy, in the first-line setting compared with ET alone in HER2-positive/HR-positive advanced breast cancer (13,14). A significant reduction in the risk of progression was demonstrated with the combination of TRAS plus anastrozole or LAP plus letrozole compared with AI monotherapy (see Table 1). The use of dual anti-HER2 inhibition represents a promising strategy to further improve the clinical outcomes of these patient population. The combination of TRAS and LAP is based on the different mechanisms of action of these drugs. TRAS is a recombinant humanized monoclonal antibody directed against the extracellular domain of HER2 whereas LAP is a HER2 and HER1 tyrosine kinase inhibitor (TKI) that binds to the intracellular phosphorylation domain to prevent receptor autophosphorylation upon ligand binding. HER2-positive Breast cancer models have demonstrated synergistic interactions between TRAS and LAP (17). In the clinic, dual anti-HER2 blockade plus chemotherapy has been shown to improve outcomes in both the neoadjuvant, adjuvant and metastatic settings compared with single HER2 blockade (16,18,19) .The present study (ALTERNATIVE) is the first large, randomized clinical trial to exclusively investigate this chemotherapy-sparing regimen in this specific population of patients who had already received prior ET, chemotherapy and TRAS neo(adjuvant) and/or first-line metastatic setting.

Full table
The investigators from the ALTERNATIVE trial concluded that: “Dual HER2 blockade with showed superior PFS benefit in patients with HER2-positive/HR-positive MBC. This combination offers an effective and safe chemotherapy-sparing alternative treatment regimen for this patient population.”
Similarly, the PERTAIN trial report benefit of dual HER2 blockade (hazard ratio =0.65) in HER-2 positive HR-positive advanced breast cancer. The median PFS was 18.9 months with pertuzumab + TRAS + AI versus 15.80 months with TRAS + AI. It is important to emphasize that this trial evaluated a population with different characteristics where the majority of patients (77%) were TRAS naïve and only 55% of patients had received induction chemotherapy (20).
The American Society of Clinical Oncology Clinical Practice Guideline strongly recommend an association of HER2-targeted therapy with chemotherapy as first-line treatment for metastatic HR-positive/HER2 positive breast cancer (21). The association of ET plus anti-HER2 agents is an option in selected cases referred as moderately strong recommendation. The ALTERNATIVE study certainly adds to this recommendation despite that no direct comparisons were performed to date between ET versus chemotherapy plus HER2-targeted therapy. The rational is that selected patients may benefit from the association of ET and dual HER2-targeted therapy and delay chemotherapy associated side effects. Of note in the ALTERNATIVE trial patients with measurable disease benefit more from dual HER2 blockage suggesting that this regimen may be an option in a more aggressive disease. In this context, the identification of predictive factors and better understanding of mechanism of resistance in HER2-positive/HR-positive should be a scientific priority (9).
In conclusion, “triple positive” breast cancers represent a distinct subtype of tumors in which specific treatment approaches that consider bidirectional crosstalk between the ER and HER2 pathways are promising. In selected patients, a chemotherapy-sparing treatment strategy using the combination of ET and dual anti-HER2 agents such as in ALTERNATIVE trial may be considered. We can foresee significant additional data in this area in the near future once several therapeutic agents targeting resistance mechanisms of ET and anti-HER treatments are currently under development.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Ming-Hui Zhang (Department of Medical Oncology, Harbin Medical University Cancer Hospital, Harbin, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.04.19). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Johnston SRD, Hegg R, Im SA, et al. Phase III, Randomized Study of Dual Human Epidermal Growth Factor Receptor 2 (HER2) Blockade With Lapatinib Plus Trastuzumab in Combination With an Aromatase Inhibitor in Postmenopausal Women With HER2-Positive, Hormone Receptor-Positive Metastatic Breast Cancer: ALTERNATIVE. J Clin Oncol 2018;36:741-8. [Crossref] [PubMed]
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82. [Crossref] [PubMed]
- Vici P, Pizzuti L, Natoli C, et al. Triple positive breast cacer: a distinct subtype? Cancer Treat Rev 2015;41:69-76. [Crossref] [PubMed]
- De Laurentiis M, Arpino G, Massarelli E, et al. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res 2005;11:4741-8. [Crossref] [PubMed]
- Lipton A, Ali S, Leitzel K, et al. Elevated serum Her-2/neu level predicts decreased response to hormone therapy in metastatic breast cancer. J Clin Oncol 2002;20:1467-72. [Crossref] [PubMed]
- Ma CX, Reinert T, Chmielewska I, et al. Mechanisms of aromatase inhibitors resistance. Nat Rev Cancer 2015;15:261-75. [Crossref] [PubMed]
- Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 2011;62:233-47. [Crossref] [PubMed]
- Osborne CK, Bardou V, Hopp TA, et al. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst 2003;95:353-61. [Crossref] [PubMed]
- Lousberg L, Collignon J, Jerusalem G. Resistance to therapy in estrogen receptor positive and human epidermal growth factor 2 positive breast cancers: progress with latest therapeutic strategies. Ther Adv Med Oncol 2016;8:429-49. [Crossref] [PubMed]
- Arpino G, Green SJ, Allred DC, et al. HER-2 amplification, HER-1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: a southwest oncology group study. Clin Cancer Res 2004;10:5670-6. [Crossref] [PubMed]
- Xia W, Bacus S, Hegde P, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci U S A 2006;103:7795-800. [Crossref] [PubMed]
- Witkiewicz AK, Cox D, Knudsen ES. CDK4/6 inhibition provides a potent adjunct to Her2-targeted therapies in preclinical breast cancer models. Genes Cancer 2014;5:261-72. [PubMed]
- Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol 2009;27:5529-37. [Crossref] [PubMed]
- Johnston S, Pippen J Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 2009;27:5538-46. [Crossref] [PubMed]
- Huober J, Fasching PA, Barsoum M, et al. Higher efficacy of letrozole in combination with trastuzumab compared to letrozole monotherapy as first-line treatment in patients with HER2-positive, hormone-receptor-positive metastatic breast cancer - results of the eLEcTRA trial. Breast 2012;21:27-33. [Crossref] [PubMed]
- von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med 2017;377:122-31. [Crossref] [PubMed]
- Alvarez RH, Hortobagyi GN. Dual human epidermal growth factor receptor 2 blockade for the treatment of HER2-positive breast cancer. Breast Cancer 2013;20:103-10. [Crossref] [PubMed]
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25-32. [Crossref] [PubMed]
- Swain SM, Kim SB, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2013;14:461-71. [Crossref] [PubMed]
- Arpino G, Ferrero JM, de la Haba-Rodriguez J, et al. Primary analysis of PERTAIN: A randomized, two-arm, open-label, multicenter phase II trial assessing the efficacy and safety of pertuzumab given in combination with trastuzumab plus an aromatase inhibitor in first-line patients with HER2-positive and hormone receptor-positive metastatic or locally advanced breast cancer. Cancer Res 2017;77:S3-04. [Crossref]
- Giordano SH, Temin S, Kirshner JJ, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2014;32:2078-99. [Crossref] [PubMed]


