
The efficacy and tolerance of high pressure oxygen combined with chemotherapy in postoperative patients with advanced gastric cancer
Introduction
The incidence of gastric cancer is increasing year by year, clinical patients are mostly diagnosed with advanced gastric cancer. The effect of surgical treatment is often limited, it is necessary to be further treated with chemotherapy, but the overall effect is still unsatisfactory. How to improve the sensitivity of cancer cells on chemotherapy drugs and simultaneously reduce the damage of chemotherapy drugs on normal tissue cells has become an important research topic. Much progress of hyperbaric oxygen on malignant tumor treatment has been made, and the sensitization mechanism that hyperbaric oxygen treats malignant tumors has been proved in the experiment, which has a good prospect in the adjuvant therapy of malignant tumor; however, the clinical application in gastric cancer after operation has not been reported. In this paper, we combined gastric cancer postoperative with hyperbaric oxygen therapy to explore the clinical application in postoperative chemotherapy for advanced gastric cancer.
Methods
The general information
A total of 56 patients from June 2014 to June 2015 with advanced gastric cancer postoperative chemotherapy were collected, and the method of random number tables was used to randomly divided 56 patients into the experimental group and the control group. There were 22 cases in the experimental group, including 10 males and 12 females, aged 40 to 79 years, and the median age was 66.4 years. In the control group, there were 23 cases, 11 males and 12 females, aged 46 to 80 years, and the median age was 64.4 years. All patients were completed gastric cancer D2 radical surgery, the cases were diagnosed with gastric cancer stage II, IIIa and IIIb by postoperative pathological histology, and assessed more than 60 points with Karnofsky Performance Status (KPS), the patients were expected to survive more than 3 months, no more than 80 years old, with normal blood picture, liver and kidney function, without vital organ lesions history of other malignancies and contraindications of chemotherapy and hyperbaric oxygen therapy. The study was approved by the Ethics Committee of Renji Hospital and informed consents were obtained from all patients. There was no statistically significant difference in general information between the two groups of patients, as shown in Table 1.

Full table
The postoperative chemotherapy regimen
D2 gastric cancer radical surgery was done by the same leading physicians, and routine chemotherapy for 3–4 weeks were done after surgery. The project of capecitabine and oxaliplatin for 3-week was adopted: carpeyabine 1,000 mg/m2 was taken orally twice a day from day 1 to day 14, and oxaliplatin 130 mg/m2 was intravenously injected for 2 hours. A period consists of 3 weeks, and there are eight periods. If serious adverse reactions occurred or markers of tumor markers are increased in patients during chemotherapy, the chemotherapy regimen will be changed according to the situation. The methoxyclopamine and dexamethasone were used to prevent nausea and vomiting during chemotherapy.
High pressure oxygen solution
Hyperbaric oxygen combined with chemotherapy were performed in the experimental groups. The medical hyperbaric oxygen chamber with pure O2 was used to treatment, the pressure is 2.0–2.5 absolute atmosphere (ATA), the patient absorbed oxygen 20 min ×4, and absorbed air discontinuously 5 min ×3. Hyperbaric oxygen is absorbed five times before chemotherapy, once a day, and then the hyperbaric oxygen and chemotherapy were performed simultaneously. The chemotherapy was started at 15–30 min after the absorption of oxygen, the pure saturated oxygen was absorbed for 1 hour after chemotherapy, the hyperbaric oxygenation was continued to absorbed 10 times and once a day during the interval chemotherapy.
Observation standards and indicators
Two groups of patients were expected to complete eight chemotherapy periods, the imaging evaluation results of two groups of patients in the experimental group and the control group were compared, the upper abdominal enhancement computed tomography (CT) were used to evaluate tumor recurrence and enlarged lymph node status, the changes of tumor indicators (CEA and CA199) in two groups of chemotherapy patients were observed, the chemotherapy adverse reaction were classified into 0 (no), I (mild), II (moderate), III (severe) and IV (heavy) according to the WHO evaluation standard. Quality of Life-Core 30 Questionnaire (QLQ-C30) developed by EORTC was performed to evaluated life quality of patients. The questionnaire was completed by the patients, and the questionnaire content was presented the patients with dyslexia by the doctor or their family members who answered the questionnaire according to the patient's answers, as the basis for the evaluation of the clinical application effect.
Statistical method
SPSS13.0 software was used to analyze data statistics. The metering information are presented as mean ± SD. Differences between means were determined by Student’s t-test with in two groups the counting data were indicated by the adoption rate (%) and the χ2 test is adopted. P<0.05 was set as a significant criterion.
Result
Comparison of general data before chemotherapy
The age, nutritional status, gender and pTNM stage of the two groups were not statistically significant (P>0.05) (Table 1).
Imaging indicators evaluation
Experimental group and control group 1-year image evaluation for recurrence or lymph node metastasis were 1 case (4.54%) and 6 cases (26.0%) respectively; the recurrence rate of the experimental group was significantly lower than that in the control group, and the difference between the two groups was statistically significant (χ2=3.972, P=0.046).
Comparison of tumor markers
The changes of tumor markers CEA and CA199 in the serum of the two groups after treatment were observed. In the period of chemotherapy, there were 7 patients in the experimental group who showed persistent abnormality of CEA, accounting for 31.8%, 9 patients in the control group, accounting for 39.1%, and no statistically significant difference between the two groups (χ2=0.262, P=0.609). During chemotherapy, there were 3 patients in the experimental group showed persistent abnormality of CA199, accounting for 13.6%, 10 patients in the control group, accounting for 43.4%, and the differences between the two groups were statistically significant (χ2=4.874, P=0.027).
Comparison of adverse reactions
There were different degrees of reaction of blood system, digestive system and peripheral nervous system in the two groups after treatment. There was a statistical difference between the experimental group and the control group in hemocytopenia, thrombocytopenia, nausea and vomiting, and peripheral nerves reaction, which was statistically significant (P<0.05). However, there was no significant difference in hemoglobin reduction and diarrhea (P>0.05) (Table 2).
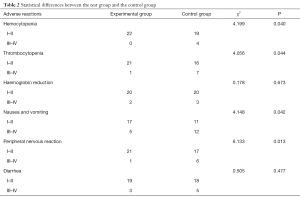
Full table
Quality of life (QOL) ratings
The QLQ-C30 score was 42.32±16.20 in the experimental group, 79.53±18.54 in the control group, the t value was 7.156, P<0.0001. The QOL in the experimental group was significantly lower than that in the control group, and the difference was statistically significant (P<0.05).
Discussion
Gastric cancer is one of the most common malignancies. It is reported by the world health organization’s “world cancer report 2014” that the number of new cases of gastric cancer and the number of deaths in China are the highest in the world. Shanghai is the region with high incidence of gastric cancer, and the incidence is increasing year by year. Patients with clinical diagnosis are mostly in advanced progress and the prognosis is poorer (1). At present, the radical operation and postoperative chemotherapy for gastric cancer is standard treatment, the study has shown that compared with separate surgery, 3-year survival rate, progression-free survival and recurrence rate were improved by postoperative adjuvant chemotherapy (2). CLASSIC study results provide important evidence for the value of postoperative adjuvant chemotherapy for gastric cancer. A total of 1.035 II–III stage gastric cancer patients after surgery were randomly assigned into the group of capecitabine combinated with oxaliplatin (XELOX project) group (n=520) or observation group (n=515), the results showed that postoperative adjuvant chemotherapy increased the survival rate from 59% to 74% (HR =0.56; 95% CI: 0.44–0.72; P<0.0001) (3). Postoperative chemotherapy is an important treatment of gastric cancer, the purpose is to control the local recurrence and eliminate tiny metastases, thus improve postoperative disease-free survival and overall survival, but the overall therapeutic effect of gastric cancer is still unsatisfactory. The unsatisfactory effect of chemotherapy in advanced gastric cancer is an important factor for postoperative recurrence, multidrug resistance of chemotherapy drugs is responsible for unideal chemotherapy effect (4). How to improve the sensitivity of cancer cells to chemotherapy drugs and reduce the damage of chemotherapy drugs to normal tissue cells has become an important research topic.
Hypoxia is one of the basic characteristics of the microenvironment of solid tumor, that the tumor tolerates hypoxia is important mechanism of enhancement ability in the tumor development and tumor transfer, it also can induce multi-drug resistance, which leads to poor chemotherapy effect (5). Hypoxia can induce gene expression related to blood vessel formation and hypoxia metabolism, and hypoxia-inducible factor-1 (HIF-1) plays a very important role (6,7). The application of hyperbaric oxygen as a sensitizer and disinfectant for malignant tumor chemotherapy has been recognized. Hyperbaric oxygen can improve oxygen levels of hypoxic cells within tumors, it enhanced the sensitivity of tumor cells to chemotherapy drugs by down-regulating the expression of HIF-1, the drug concentration in the tumor was increased. In this way, the effect of hyperbaric oxygen on the sensitization of chemotherapy was achieved (8). In this study, 1-year image evaluation for recurrence or lymph node metastasis in experimental group and control group are 1 case (4.54%) and 6 cases (26.0%) respectively, the recurrence rate in the experimental group was significantly lower than that in the control group, and the difference between the two groups was statistically significant (χ2=3.972, P=0.046). Hyperbaric oxygen combined with chemotherapy can reduce tumor recurrence in the near future and prolonged the patient’s survival period, which could be applied to clinical practice.
Postoperative adjuvant chemotherapy of gastric cancer is efficient in clinic, but it is accompanied by some problems. The main problem is associated complications caused by chemotherapy, such as different degree adverse reactions of the digestive system, blood system, nervous system, chemotherapeutic drugs cause the complications occurrence of the hemocytopenia, haemoglobin reduction and thrombocytopenia resulted by myelosuppression, nausea and vomiting, diarrhea, hand foot syndrome and so on (9,10). The adverse reaction of chemotherapy was significantly reduced after the application of hyperbaric oxygen, for instance, myelosuppression, gastrointestinal reaction and hair loss in hyperbaric oxygen group improved markedly. The treatment of hyperbaric oxygen combined with chemotherapy has improved the efficacy of chemotherapy while reducing the adverse reaction of chemotherapy, which has been proved in experiments (11,12). However, the clinical application in postoperative gastric cancer has not been reported. There was a statistical difference between the experimental group and the control group in the hemocytopenia thrombocytopenia, nausea and vomiting, and peripheral nervous reaction (P<0.05), but there was no significant difference in hemoglobin reduction and diarrhea (P>0.05). Meanwhile, QLQ-C30 was performed to compare the life quality score in two groups. EORTCQLQ-C30 scale is used widely in the world, which can reflect the multidimensional structure of the life quality, it is applicable to determine the life quality of cancer patients in China (13). The results showed that the QLQ-C30 score of the experimental group was 42.32±16.20, the control group was 79.53±18.54, and the score of life quality in the experimental group was significantly lower than that in the control group, the QOL in the experimental group was significantly improved.
Conclusions
In this study, the application of hyperbaric oxygen combined with chemotherapy in the treatment of postoperative gastric cancer reduced the recent local recurrence rate and the side reaction, the safety, tolerance of chemotherapy and postoperative survival time of patients were markedly improved. The small sample is the main deficiency in the study, so it is not sufficient to evaluate the effect factors of postoperative adjuvant chemotherapy. Long-term survival was not assessed the follow-up time was not long enough. The long-term survival rate was not assessed as the follow-up time was not long enough. Further information was needed to improve the analysis of clinical efficacy and tolerance in postoperative chemotherapy patients and the specific mechanism in clinical application still needs to be further investigated.
Acknowledgments
Funding: This study was supported by grants from the Shanghai Municipal Commission of Health and Family Planning (grant number: 201440523).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.04.16). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Renji Hospital [(2014) 120K] and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zou WB, Yang F, Li ZS. How to improve the diagnosis rate of early gastric cancer in China. Zhejiang Da Xue Xue Bao Yi Xue Ban 2015;44:9-14. [PubMed]
- Ajani J. Review of capecitabine as oral treatment of gastric, gastroesophageal, and esophageal cancers. Cancer 2006;107:221-31. [Crossref] [PubMed]
- Noh SH, Park SR, Yang HK, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:1389-96. [Crossref] [PubMed]
- Shang Y, Feng B, Zhou L, et al. The miR27b-CCNG1-P53-miR-508-5p axis regulates multidrug resistance of gastric cancer. Oncotarget 2016;7:538-49. [Crossref] [PubMed]
- Merritt WM, Sood AK. Markers of angiogenesis in ovarian cancer. Dis Markers 2007;23:419-31. [Crossref] [PubMed]
- Menrad H, Werno C, Schmid T, et al. Roles of hypoxia-inducible factor-1alpha (HIF-1alpha) versus HIF-2alpha in the survival of hepatocellular tumor spheroids. Hepatology 2010;51:2183-92. [Crossref] [PubMed]
- Huang C, Sun Z, Sun Y, et al. Association of increased ligand cyclophilin A and receptor CD147 with hypoxia, angiogenesis, metastasis and prognosis of tongue squamous cell carcinoma. Histopathology 2012;60:793-803. [Crossref] [PubMed]
- Nakamura J, Kitajima Y, Kai K, et al. HIF-1alpha is an unfavorable determinant of relapse in gastric cancer patients who underwent curative surgery followed by adjuvant 5-FU chemotherapy. Int J Cancer 2010;127:1158-71. [Crossref] [PubMed]
- Alcindor T, Beauger N. Oxaliplatin: a review in the era of molecularly targeted therapy. Curr Oncol 2011;18:18-25. [Crossref] [PubMed]
- Montagnani F, Turrisi G, Marinozzi C, et al. Effectiveness and safety of oxaliplatin compared to cisplatin for advanced, unresectable gastric cancer: a systematic review and meta-analysis. Gastric Cancer 2011;14:50-5. [Crossref] [PubMed]
- Takiguchi N, Saito N, Nunomura M, et al. Use of 5-FU plus hyperbaric oxygen for treating malignant tumors: evaluation of antitumor effect and measurement of 5-FU in individual organs. Cancer Chemother Pharmacol 2001;47:11-4. [Crossref] [PubMed]
- Moen I, Tronstad KJ, Kolmannskog O, et al. Hyperoxia increases the uptake of 5-fluorouracil in mammary tumors independently of changes in interstitial fluid pressure and tumor stroma. BMC Cancer 2009;9:446. [Crossref] [PubMed]
- Scott NW, Fayers PM, Bottomley A, et al. Comparing translations of the EORTC QLQ-C30 using differential item functioning analyses. Qual Life Res 2006;15:1103-15; discussion 1117-20. [Crossref] [PubMed]


